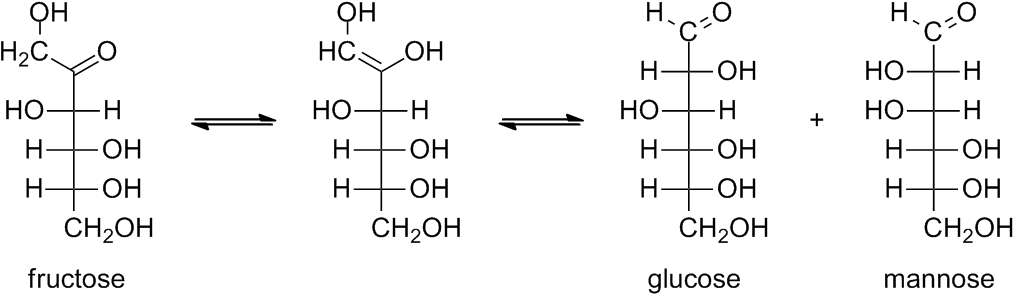
I don't understand how to tell the difference between reducing and nonreducing sugars when looking at the glycosidic bonds between different molecules. I also seem to struggle with being able to recognize where the anomeric carbon is. Maybe this is the problem? Can anyone help me out please?
here is a question, i continue to struggle with:
-------------------------------------------------------
Raffinose can be described as a:
A. reducing sugar
B. nonreducing sugar
C. disaccharide
D. glycoprotein
B) nonreducing sugar
As shown by the molecular structure, raffinose is clearly not a disaccharide. It is considered to be olgiosaccharide (or even a polysaccharide). Since there are no amino acids attached to and of the three sugar residues, it cannot be a glycoprotein. This means that raffinose is either a reducing sugar or a non-reducing sugar.
Sugars having anomeric carbon atoms that have not formed glycosides (containing an acetal linkage) are called reducing sugars. Recall that the cycli and linear forms of both aldoses and ketoses are readily interconverted. The aldehyde function in galactose and glucose can easily be oxidized by an oxidizing agent like Benedict's reagent (a solution of copper(II) sulfate and sodium citrate in aq base) to the corresponding carboxylic acid (see below)
Fructose is an alpha-hydroxy ketone and alpha-hydroxy ketones are easily oxidized to the diketone by Benedict's reagent. The Benedict's reagent, which is a blue solution, is reduced to a red precipitate. If the aldehyde and ketone functional groups remain tied up in a glycosidic bond (as shown in the structure of raffinose), then they cannot react with the Benedict's reagent.
here is a question, i continue to struggle with:
-------------------------------------------------------
Raffinose can be described as a:
A. reducing sugar
B. nonreducing sugar
C. disaccharide
D. glycoprotein
B) nonreducing sugar
As shown by the molecular structure, raffinose is clearly not a disaccharide. It is considered to be olgiosaccharide (or even a polysaccharide). Since there are no amino acids attached to and of the three sugar residues, it cannot be a glycoprotein. This means that raffinose is either a reducing sugar or a non-reducing sugar.
Sugars having anomeric carbon atoms that have not formed glycosides (containing an acetal linkage) are called reducing sugars. Recall that the cycli and linear forms of both aldoses and ketoses are readily interconverted. The aldehyde function in galactose and glucose can easily be oxidized by an oxidizing agent like Benedict's reagent (a solution of copper(II) sulfate and sodium citrate in aq base) to the corresponding carboxylic acid (see below)
Fructose is an alpha-hydroxy ketone and alpha-hydroxy ketones are easily oxidized to the diketone by Benedict's reagent. The Benedict's reagent, which is a blue solution, is reduced to a red precipitate. If the aldehyde and ketone functional groups remain tied up in a glycosidic bond (as shown in the structure of raffinose), then they cannot react with the Benedict's reagent.