- Joined
- Feb 5, 2006
- Messages
- 246
- Reaction score
- 0
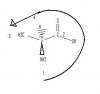
(S)-2-Aminopropanoic acid
can someone explain to me why this molecule is S and not R?
I couldn;t cut the picture out of my powerpoint slides but it looks like this
Ch3 - C - COOH
l
NH2
the C - NH2 bond is pointing at us. and the NH2 isnt attached to the CH3 but the C
Thanks for your time.
can someone explain to me why this molecule is S and not R?
I couldn;t cut the picture out of my powerpoint slides but it looks like this
Ch3 - C - COOH
l
NH2
the C - NH2 bond is pointing at us. and the NH2 isnt attached to the CH3 but the C
Thanks for your time.