Hello,
Would appreciate if anyone can help with these two questions.
1. Compound T has a melting point of 78 °C and a boiling point of 134 °C. T is soluble in water and its solution does not conduct electricity. T has covalent bonding and has a simple molecular structure.
Which property of T is not usually associated with its bonding and structure?
A the melting point
B the boiling point
C the solubility in water
D the lack of conductivity of the solution
I don’t understand why C. is the answer, when entropy and polarity of a substance affect its solubility in water, and both of these are related to the structure of the molecule.
2.
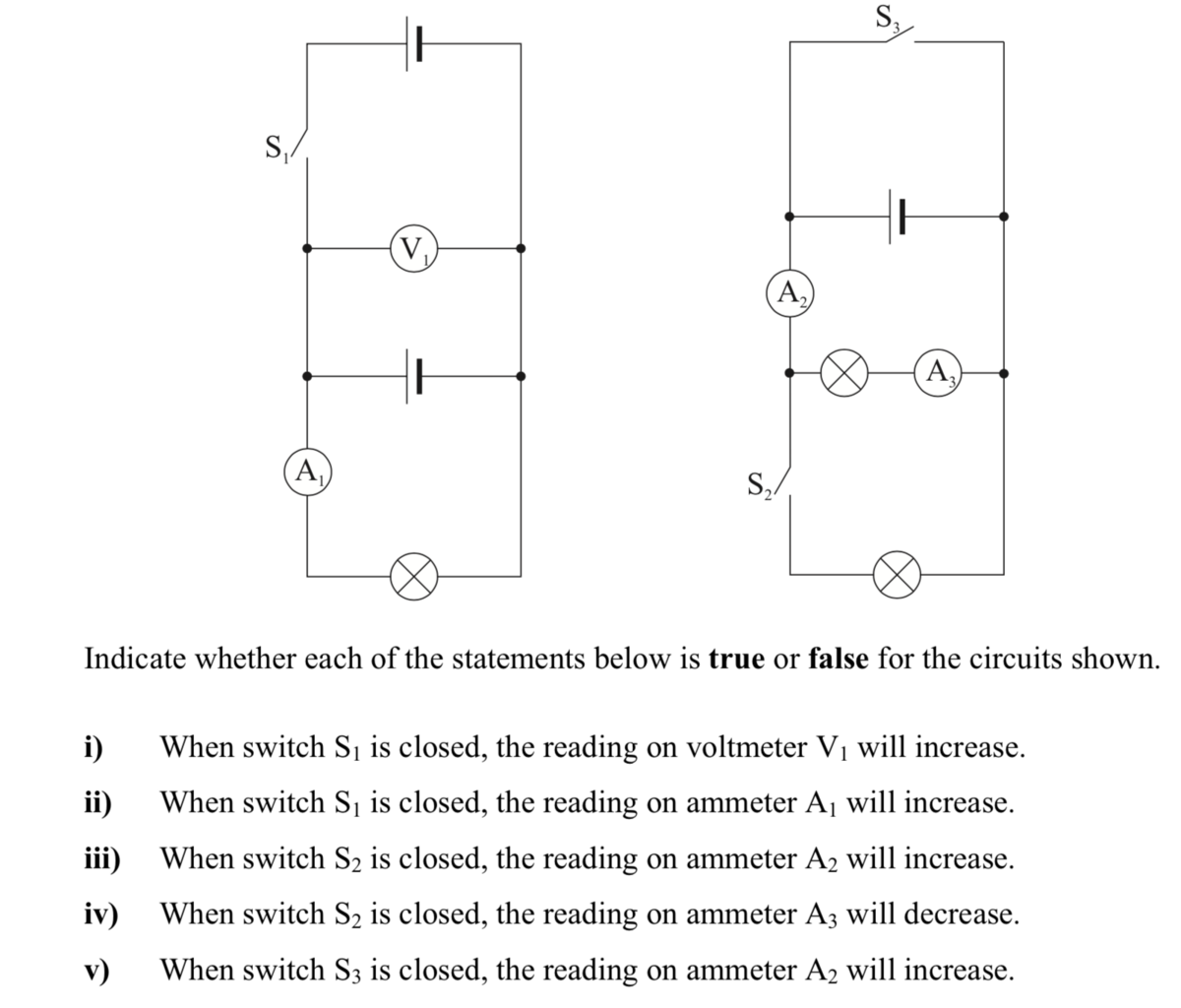
I just would like to verify if when S2 is closed, what happens to A3. So it seems the total resistance is decreased when S2 is closed. That means current in A2 will increase, but current in A3 will be the same right?
3.
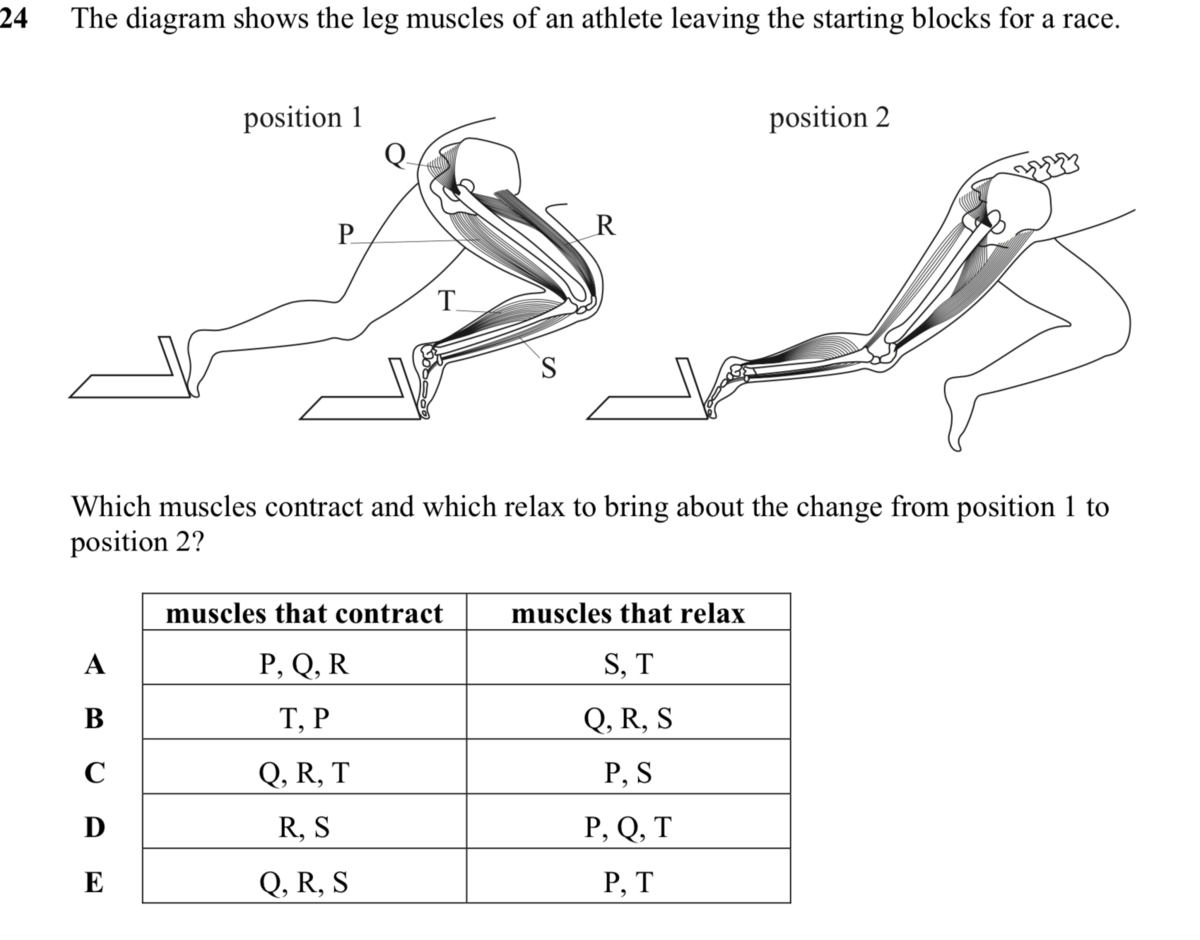
Could someone explain the reasoning behind answering this question? The answer is C.
Thank you.
Would appreciate if anyone can help with these two questions.
1. Compound T has a melting point of 78 °C and a boiling point of 134 °C. T is soluble in water and its solution does not conduct electricity. T has covalent bonding and has a simple molecular structure.
Which property of T is not usually associated with its bonding and structure?
A the melting point
B the boiling point
C the solubility in water
D the lack of conductivity of the solution
I don’t understand why C. is the answer, when entropy and polarity of a substance affect its solubility in water, and both of these are related to the structure of the molecule.
2.
I just would like to verify if when S2 is closed, what happens to A3. So it seems the total resistance is decreased when S2 is closed. That means current in A2 will increase, but current in A3 will be the same right?
3.
Could someone explain the reasoning behind answering this question? The answer is C.
Thank you.
Last edited:
