- Joined
- Oct 12, 2009
- Messages
- 293
- Reaction score
- 66
Check out all the studies that are done on BurstDR and HF10 besides the Senza and Sunburst study.
If you have a thought of using two systems with an option of tonic then Abbott with BurstDR and tonic fits.
Supposedly Senza tonic is not great.
Burst other than BurstDR is cluster tonic and does not light up the medial pathway. This was studied
HF10 is only used with Nevro. This was Studied
1k,4K, 7k, 10k was studied in the Proco study.
Whisper study was very lacking.
Now when you talk about randomness and something not studied well at all then go ahead and try something like targeting multiple stimulation patterns at a time.
I don’t care about these companies but I do care about OUR field.
When we fall for gimmicks and not research at this point then we are taking steps backwards.
They are all gimmicky, that is what sells. You can’t just buy into it, but . A lot of this sounds like what the Abbott reps say to me.
I don’t think they’ve proven that the fact their burst doesn’t fully repolarize matters in outcomes, just “mimics neuronal firing”. Sounds good but sounds like a gimmick too.
Medial pathways, sure it makes intuitive sense but does it matter? All the fMRI studies I take with a grain of salt.
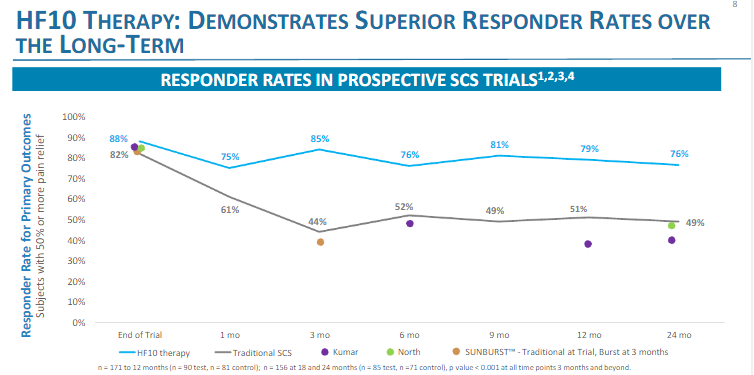
The SUNBURST study showed crappy endpoints for both their tonic and burst patients, less than 50% improvement in VAS at 12 weeks. Wouldn’t even qualify for an implant if they weren’t already implanted. I’d hate to see them at 12 months... if anything this made me not want to use Abbott.
SENZA was great, I can’t say anything poorly about someone using me to based upon that study. This is the “best” device on the market from a study standpoint at this time, in my opinion. But again, older system was compared with substantial change in the software in pretty much all competitors at this point. Not studied against so it’s hard to say it matters, but I believe that there is a substantial difference in the software.