- Joined
- Jan 16, 2012
- Messages
- 1,095
- Reaction score
- 972
- Points
- 5,491
- Resident [Any Field]
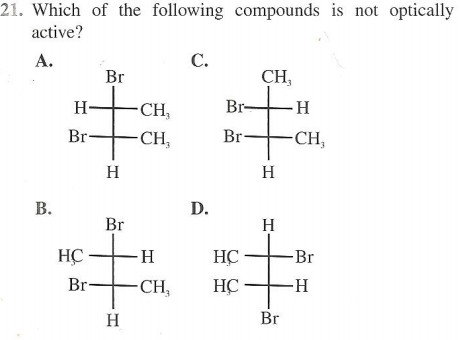
I have no idea what I'm screwing up. I thought that in order for it to be optically active it needed to be chiral, meaning that there was at least one chiral center in the molecule. Chiral center would mean that there are four different groups attached to a carbon, but I don't see that not happening in any of the choices given? I'm sure this is really easy and I'm just being dumb, but some help would be great. Thanks!
