- Joined
- Apr 15, 2009
- Messages
- 88
- Reaction score
- 2
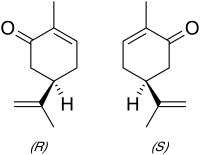
So I came across a question that is asking what is the absolute configuration of (+) Carvone and (-) Carvone respectively ? I thought + means clockwise = R and (-) = Counter = S so I chose R,S and the book said wrong it's S and R what did I miss here? did I just misread the question