- Joined
- Jun 29, 2004
- Messages
- 203
- Reaction score
- 2
I was given the below reaction and asked about whether its sn2 or sn1. I'm having trouble understanding the provided explanation
the only alkly halide in that reaction is the Ch3X and its the Nucleophile, I'm confused why they are referring to it as the Electrophile
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~`
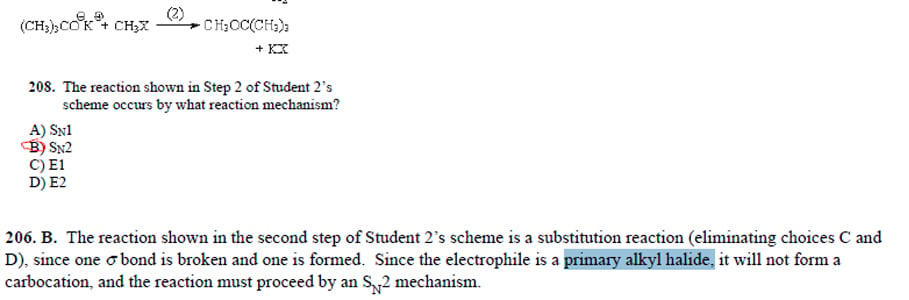
the only alkly halide in that reaction is the Ch3X and its the Nucleophile, I'm confused why they are referring to it as the Electrophile
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~`

Last edited:
