- Joined
- Oct 4, 2017
- Messages
- 5,445
- Reaction score
- 10,683
- Points
- 5,711
- Attending Physician
Advertisement - Members don't see this ad
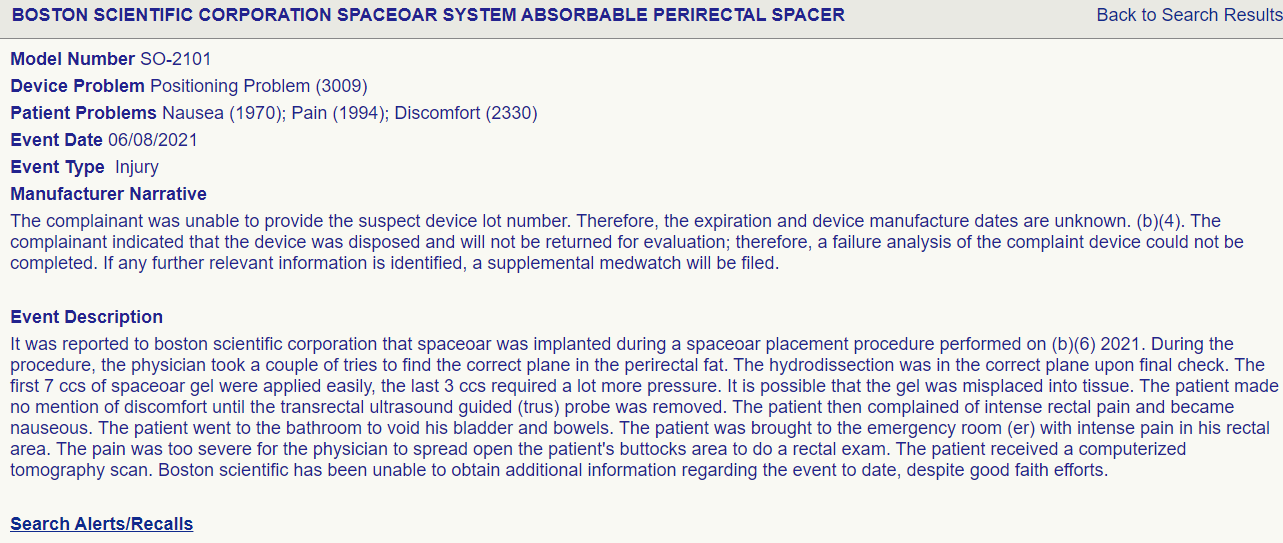
regarding space oar, one DEATH was reported: we should continue to check the MAUDE database. Almost certainly underestimates number of true vs reported events. There were basically no events on the trial? Sketchy AF
 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
"To accomplish this, on May 15, 2020, the MAUDE database, which houses medical device reports submitted to the FDA by mandatory and voluntary reporters, was accessed via a publicly available online interface and events were searched for from May 1, 2015, to May 1, 2020, using the search term “SpaceOAR”. The description of each event was reviewed and scored by two independent radiation oncologists (WAH and CAFL). The results were then compared collectively, and a final adjudication of scored toxicity events was created. A total of 85 reported events in the MAUDE database were available for review. Among these, 80 (94%) had event descriptions that could be characterised using the Common Terminology Criteria for Adverse Events (version 5.0). Of the toxicity events, 59 (69%) were grade 3, 4, or 5. 20 (24%) were grade 4 events, including multiple independent descriptions of colostomy, anaphylactic events, rectal wall injection, or pulmonary emboli requiring hospital admission. One death was reported. The toxicity score distribution can be seen in the appendix (p 2).
Considering benefit and risk before routinely recommending SpaceOAR - PMC
"To accomplish this, on May 15, 2020, the MAUDE database, which houses medical device reports submitted to the FDA by mandatory and voluntary reporters, was accessed via a publicly available online interface and events were searched for from May 1, 2015, to May 1, 2020, using the search term “SpaceOAR”. The description of each event was reviewed and scored by two independent radiation oncologists (WAH and CAFL). The results were then compared collectively, and a final adjudication of scored toxicity events was created. A total of 85 reported events in the MAUDE database were available for review. Among these, 80 (94%) had event descriptions that could be characterised using the Common Terminology Criteria for Adverse Events (version 5.0). Of the toxicity events, 59 (69%) were grade 3, 4, or 5. 20 (24%) were grade 4 events, including multiple independent descriptions of colostomy, anaphylactic events, rectal wall injection, or pulmonary emboli requiring hospital admission. One death was reported. The toxicity score distribution can be seen in the appendix (p 2).
Last edited:


 Help
Help