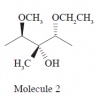
Is the last stereocenter (the one on the very right) R or S?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Stereocenters
- Thread starter NA19
- Start date
- Joined
- May 26, 2013
- Messages
- 334
- Reaction score
- 2
R I think because the H is coming out of the page
This was actually something that I was planning on looking up to double check
someone clarify for me if i'm wrong
This was actually something that I was planning on looking up to double check
someone clarify for me if i'm wrong
- Joined
- May 26, 2010
- Messages
- 244
- Reaction score
- 2
R I think because the H is coming out of the page
This was actually something that I was planning on looking up to double check
someone clarify for me if i'm wrong
yeah i agree. it'd be S if the H was going into the page
But it doesn't even show the H? I thought it would be S because priority #1 is the OCH2CH3 group, priority #2 is the C with the OH attached, and then priority #2 is the CH3 group. So it goes counter clockwise.
- Joined
- May 26, 2010
- Messages
- 244
- Reaction score
- 2
But it doesn't even show the H? I thought it would be S because priority #1 is the OCH2CH3 group, priority #2 is the C with the OH attached, and then priority #2 is the CH3 group. So it goes counter clockwise.
Doesn't need to show the H, we know it's there. It would be S, but since the H isn't going going into the page (by convention), then it is R.
- Joined
- Jul 18, 2012
- Messages
- 4,407
- Reaction score
- 2,232
I Agree, I got R too.

- Joined
- May 26, 2010
- Messages
- 244
- Reaction score
- 2
It will be R. As everybody else said.Is the last stereocenter (the one on the very right) R or S?
Doesn't need to show the H, we know it's there. It would be S, but since the H isn't going going into the page (by convention), then it is R.
So if the H isn't shown, do you assume it's coming out of the page?
That depends; usually the main carbon chain is in the plane of the page and substituents are sticking in/out of the plane. If a substituent is pointing forwards with a wedge bond, you assume the hydrogen is backwards with a dashed bond, and vice versa. So it all depends on the depiction of the more important group.So if the H isn't shown, do you assume it's coming out of the page?
Similar threads
- Replies
- 5
- Views
- 2K