hey guys if someone could help me out with two of these questions id really appreciate it!
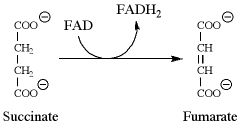
104.If a population of succinyl-CoA molecules were radioactively labeled with 14C at the C-1 carbonyl carbon, then in a population of fumarate molecules derived from those labeled precursors, the label would be found only at the:
A. C-1 carboxyl carbon atom.
B. C-2 methylene carbon atom.
C. C-1 and C-4 carboxyl carbon atoms.
C is the best answer. Look at the reaction in Figure 3 of the passage, and label the C-1 carbon atom of succinyl-CoA. Once succinate is formed, this label could end up on either one of the carboxylate carbon atoms. Note, however, that the label does not end up on both carboxylate carbon atoms of succinate at the same time. The label is found on one carboxylate carbon atom or the other (i.e., either the C-1 or C-4 carbon atom). This is because succinate is a symmetrical molecule. We now have a labeled succinate molecule (on either C-1 or C-4), and we convert that molecule into fumarate as outlined in the diagram supplied with the question. Since the carbon atoms do not change, we still would find that the label is on either the C-1 or C-4 carbon atom. The best answer is C.
D. C-2 and C-3 methylene carbon atoms.
127.By convention, peptides and proteins are drawn with the N-terminus on the left and the C-terminus on the right. However, it is not always apparent which is the N-terminus and which is the C-terminus of certain small peptides. The structure shown below is for thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus.
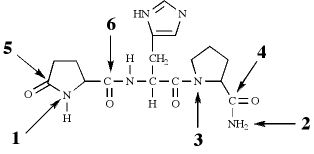
The N-terminus and C-terminus, respectively, are MOST likely to be the sites indicated by which pair of arrows?
A. Arrows 3 and 5
B. Arrows 2 and 5
C. Arrows 1 and 4
C is the best answer. At first glance, the N-terminus and the C-terminus of TRH are not readily recognizable, because they both exist as amides rather than their more discernable typical forms. Let's consider some basic concepts first. We see four amide bonds and two peptide bonds in the structure. Suppose we hydrolyze all those amide bonds that are not peptide bonds. Our resulting tripeptide would look like what is shown below (on the right).
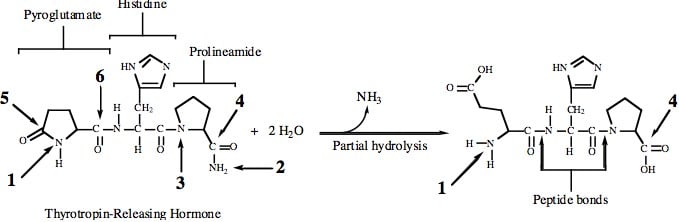
It now becomes clear that the N-terminus is represented by Arrow 1, while the C-terminus is represented by Arrow 4. If we were to hydrolyze TRH completely, we would get ammonia (NH3), glutamate, histidine, and proline. There would be four products. In TRH, the carboxyl side chain of the amino acid glutamate has reacted with the α-amino group. This structure is referred to as pyroglutamate. Also, since there is an amino group at the C-terminus of TRH, we would refer to the amino acid proline as prolineamide. You are not expected to know this, but it might help you to find the best answer more rapidly, if you do.
You could have also solved this question by focusing on α-carbons. The α-carbon lies between the amino nitrogen and carbonyl carbon of an amino acid residue. The five-member ring on the left end of TRH has its α-carbon between the nitrogen corresponding to arrow 1 and the carbonyl carbon corresponding to arrow 6. The five-member ring on the right end of TRH has its α-carbon between the nitrogen corresponding to arrow 3 and the carbonyl carbon corresponding to arrow 4. There is no other α-carbon after carbonyl carbon 4, so that must be the C-terminus. Knowing that the right end of TRH is the C-terminus tells us that the N-terminus, bonded to the α-carbon of the five-member ring on the left side, must be the N pointed to by arrow 1. The best answer is C.
First thing is that i thought peptide bonds were made up by amide bonds. This whole question really rattled me. The explanation was really confusing. If someone can help me out, it would be much appreciated!
104.If a population of succinyl-CoA molecules were radioactively labeled with 14C at the C-1 carbonyl carbon, then in a population of fumarate molecules derived from those labeled precursors, the label would be found only at the:

A. C-1 carboxyl carbon atom.
B. C-2 methylene carbon atom.
C. C-1 and C-4 carboxyl carbon atoms.
C is the best answer. Look at the reaction in Figure 3 of the passage, and label the C-1 carbon atom of succinyl-CoA. Once succinate is formed, this label could end up on either one of the carboxylate carbon atoms. Note, however, that the label does not end up on both carboxylate carbon atoms of succinate at the same time. The label is found on one carboxylate carbon atom or the other (i.e., either the C-1 or C-4 carbon atom). This is because succinate is a symmetrical molecule. We now have a labeled succinate molecule (on either C-1 or C-4), and we convert that molecule into fumarate as outlined in the diagram supplied with the question. Since the carbon atoms do not change, we still would find that the label is on either the C-1 or C-4 carbon atom. The best answer is C.
D. C-2 and C-3 methylene carbon atoms.
127.By convention, peptides and proteins are drawn with the N-terminus on the left and the C-terminus on the right. However, it is not always apparent which is the N-terminus and which is the C-terminus of certain small peptides. The structure shown below is for thyrotropin-releasing hormone (TRH), which is produced by the hypothalamus.

The N-terminus and C-terminus, respectively, are MOST likely to be the sites indicated by which pair of arrows?
A. Arrows 3 and 5
B. Arrows 2 and 5
C. Arrows 1 and 4
C is the best answer. At first glance, the N-terminus and the C-terminus of TRH are not readily recognizable, because they both exist as amides rather than their more discernable typical forms. Let's consider some basic concepts first. We see four amide bonds and two peptide bonds in the structure. Suppose we hydrolyze all those amide bonds that are not peptide bonds. Our resulting tripeptide would look like what is shown below (on the right).

It now becomes clear that the N-terminus is represented by Arrow 1, while the C-terminus is represented by Arrow 4. If we were to hydrolyze TRH completely, we would get ammonia (NH3), glutamate, histidine, and proline. There would be four products. In TRH, the carboxyl side chain of the amino acid glutamate has reacted with the α-amino group. This structure is referred to as pyroglutamate. Also, since there is an amino group at the C-terminus of TRH, we would refer to the amino acid proline as prolineamide. You are not expected to know this, but it might help you to find the best answer more rapidly, if you do.
You could have also solved this question by focusing on α-carbons. The α-carbon lies between the amino nitrogen and carbonyl carbon of an amino acid residue. The five-member ring on the left end of TRH has its α-carbon between the nitrogen corresponding to arrow 1 and the carbonyl carbon corresponding to arrow 6. The five-member ring on the right end of TRH has its α-carbon between the nitrogen corresponding to arrow 3 and the carbonyl carbon corresponding to arrow 4. There is no other α-carbon after carbonyl carbon 4, so that must be the C-terminus. Knowing that the right end of TRH is the C-terminus tells us that the N-terminus, bonded to the α-carbon of the five-member ring on the left side, must be the N pointed to by arrow 1. The best answer is C.
First thing is that i thought peptide bonds were made up by amide bonds. This whole question really rattled me. The explanation was really confusing. If someone can help me out, it would be much appreciated!
