B is correct. From point b to c in the diagram in Figure 1, the volume does not change, so no work can be done. Work is defined as -PdeltaV, so a change in volume(deltaV) of zero means that PdeltaV is zero and thus no work is done. The best answer is therefore choice B. In choices A, C,and D,there is work being done,and that is not possible at constant volume.
okay so looking at the graph it makes sense that since there is no deltaV then deltaE = q - P(0)
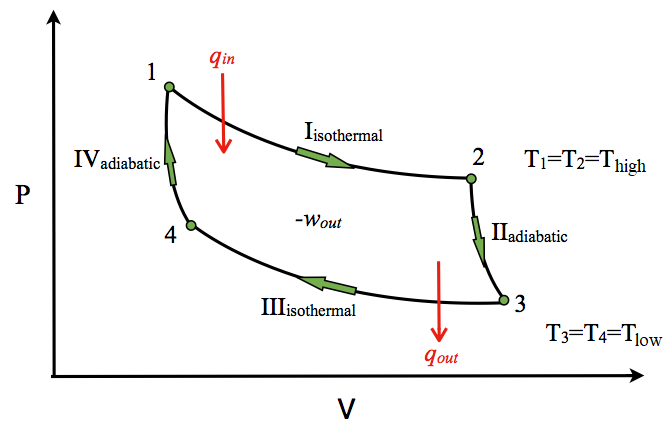
but in non ideal carnot engines that step is adiabatic expansion of gas meaning there is no change in heat so work done on the system is only PV.
looking at the ideal, carnot cycles arent isothermal and adiabatic but rather isobaric and isochoric? Meaning that step a->b is only PV, and b->c is q? can anyone think of how to apply this to an example?
thanks guys, sorry if this sounds really convoluted, ive been sitting here for hours trying to wrap my head around this