- Joined
- Apr 29, 2011
- Messages
- 2,171
- Reaction score
- 863
Are vapor pressure and partial pressure different in this scenario?
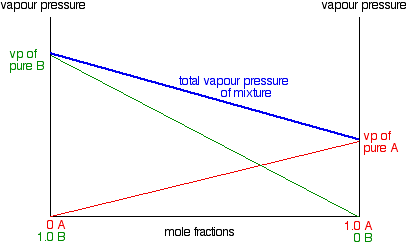
TBR GenChem Book II page 105 #42
You start off with a mixture of 2-propanoal with acetophenone.
"Addition of another 60g of 2-propanal to the original mixture wold affect the vapor pressure of aceteophenone in what way?
Answer: It would decrease the vapor pressure of aceteophenone
TBR's explanation says that because the mole fraction of acetophenone decreases, the vapor pressure of acetophenone decreases.
What about the partial pressure of acetophenone? Is that constant or does that decrease as well?
TBR GenChem Book II page 105 #42
You start off with a mixture of 2-propanoal with acetophenone.
"Addition of another 60g of 2-propanal to the original mixture wold affect the vapor pressure of aceteophenone in what way?
Answer: It would decrease the vapor pressure of aceteophenone
TBR's explanation says that because the mole fraction of acetophenone decreases, the vapor pressure of acetophenone decreases.
What about the partial pressure of acetophenone? Is that constant or does that decrease as well?