- Joined
- Mar 15, 2014
- Messages
- 403
- Reaction score
- 107
Hey guys
I'm having difficulty understanding one of the questions about Terpenes
Which of the following compounds requires the formation of three new carbon-carbon bonds when synthesized from isopentenyl pyrophosphate? (See attached photo)
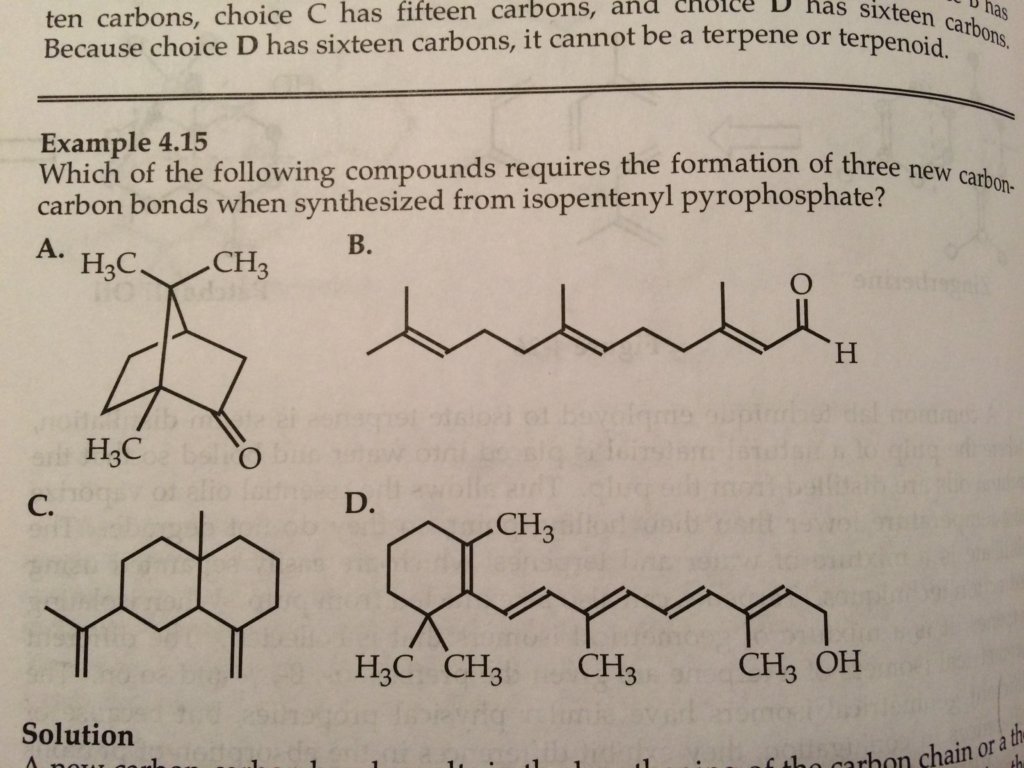
The answer is A. The solution states "In choice A, there are ten carbons and two rings. One new carbon-carbon bond is necessary to make a ten-carbon species and two more new carbon-carbon bonds are required to form the bicyclic system, so Choice A requires three carbon-carbon bonds when formed from 5-carbon isoprene derivatives."
I don't really understand what they said in the solution. If we start with a 5-carbon isoprene, then 3 additional carbon-carbon bonds will result in an 11 carbon structure (2 carbons per carbon-carbon structure times 3= 6 carbons. 5 carbons isoprene plus 6 carbons = 11 carbons).
Terpenes are pretty difficult subject for me so any help would be much appreciated.
I'm having difficulty understanding one of the questions about Terpenes
Which of the following compounds requires the formation of three new carbon-carbon bonds when synthesized from isopentenyl pyrophosphate? (See attached photo)
The answer is A. The solution states "In choice A, there are ten carbons and two rings. One new carbon-carbon bond is necessary to make a ten-carbon species and two more new carbon-carbon bonds are required to form the bicyclic system, so Choice A requires three carbon-carbon bonds when formed from 5-carbon isoprene derivatives."
I don't really understand what they said in the solution. If we start with a 5-carbon isoprene, then 3 additional carbon-carbon bonds will result in an 11 carbon structure (2 carbons per carbon-carbon structure times 3= 6 carbons. 5 carbons isoprene plus 6 carbons = 11 carbons).
Terpenes are pretty difficult subject for me so any help would be much appreciated.
