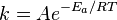- Joined
- Feb 26, 2007
- Messages
- 339
- Reaction score
- 2
Ok. So I'm a bit confused on what affects the rate of a reaction.
I know that the rate of the reaction is dependent on 3 things,
1. the energy of the collision
2. the frequency of the collision
3. orientation of the collision
so from these 3 things I can assume that temperature, catalyst, pressure and concentration will affect the rates of a reaction among other more ambiguous things.
But the question I have is, what will change the rate constant, the little k in front of the [] in the rate law WITHOUT affecting the order of the reaction. I've always assumed that temperature, pressure, and catalyst would but at this point I'm no longer sure. Do those things change the rate constant without affecting the order of the reaction or do they Affect them? Can anyone please clarify? thanks.
I know that the rate of the reaction is dependent on 3 things,
1. the energy of the collision
2. the frequency of the collision
3. orientation of the collision
so from these 3 things I can assume that temperature, catalyst, pressure and concentration will affect the rates of a reaction among other more ambiguous things.
But the question I have is, what will change the rate constant, the little k in front of the [] in the rate law WITHOUT affecting the order of the reaction. I've always assumed that temperature, pressure, and catalyst would but at this point I'm no longer sure. Do those things change the rate constant without affecting the order of the reaction or do they Affect them? Can anyone please clarify? thanks.