- Joined
- May 23, 2014
- Messages
- 265
- Reaction score
- 37
- Points
- 4,721
- Location
- Sintra, Portugal/Rio de Janeiro
- Pre-Medical
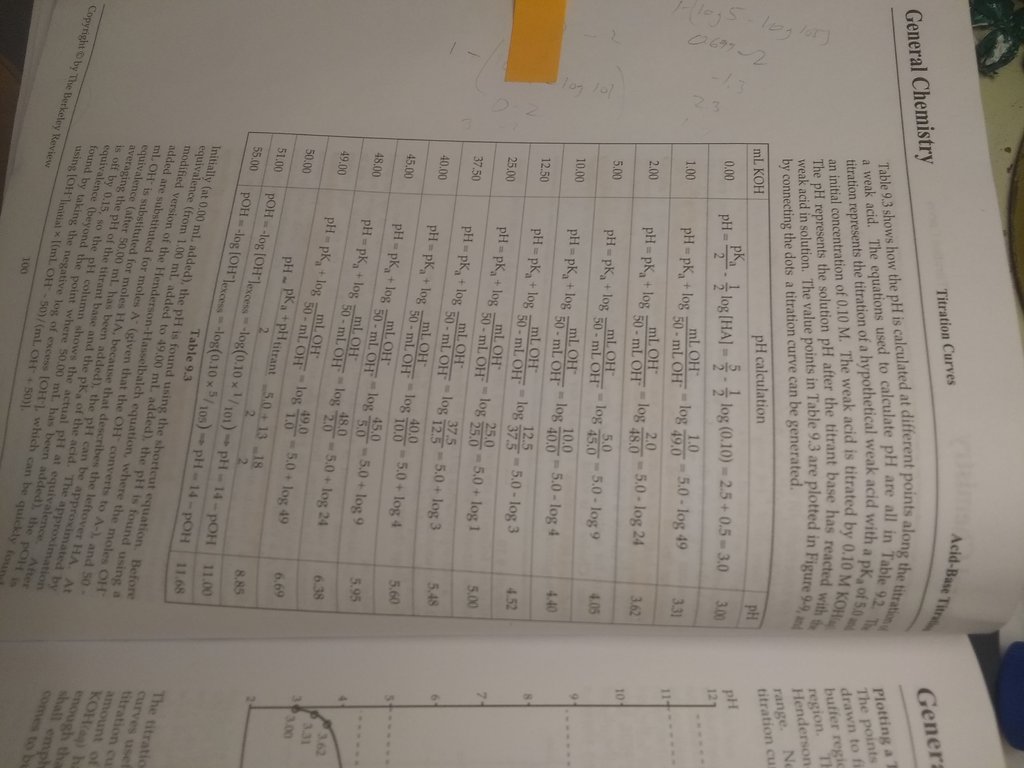
Hey guys, this is from TBR. Why is 50 mL used as the volume of strong base required to reach the equivalence point of the titration of a weak acid?