its not man. it an unfamiliar topic, yes, but you know more than enough to answer it all.
in 1, a carbocation forms after the initial SN2. Theres no carbocation in the product, but rather a double bond. Whats the only real reaction (on the MCAT anyways) that makes a double bond from a carbocation? an E1.
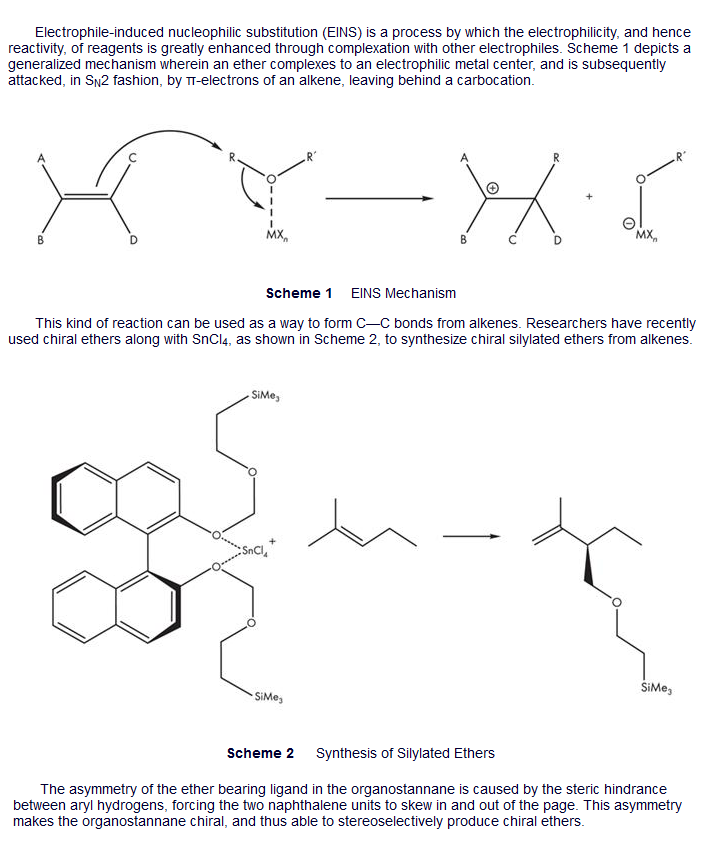
in 2, the passage says the product chirality comes from the chirality of the organostannane. And the organostannane gets its chirality from the steric hindrance of the hydrogens facing each other.
Well in the molecule in question 2, there is no steric hindrance since the rings are pointing away. Thus the compound isn't chiral and won't lead to one particular enantiomer over the other. If it makes both enantiomers of the product equally, then you'd get a racemic mixture.




 It's fixed now.
It's fixed now.