- Joined
- Dec 10, 2013
- Messages
- 474
- Reaction score
- 355
Which pair of formulas represents the empirical and molecular formulas of uracil, respectively?
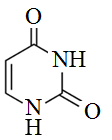
A) CHNO and C4H4N2O2
B) C2H2N2O2 and C2H2N2O2
C) C4H4N2O2 and C4H4N2O2
D) C2H2N2O2 and C4H4N2O2
I thought the answer should be C2H2NO and C4H4N2O2..... what am I missing?! I feel like an idiot right now. @Altius Premier Tutor
SOLUTION:
D is the correct answer. Counting the number of hydrogens, nitrogens, and oxygens in the molecule of uracil the molecular formula is C4H4N2O2. Since each of these numbers can be divided by two the empirical formula must be C2H2N2O2 making D the correct answer. Answer A is not correct as the empirical formula is incorrect. Answer B is showing both options as empirical formulas. Answer C gives both options as the molecular formula.

A) CHNO and C4H4N2O2
B) C2H2N2O2 and C2H2N2O2
C) C4H4N2O2 and C4H4N2O2
D) C2H2N2O2 and C4H4N2O2
I thought the answer should be C2H2NO and C4H4N2O2..... what am I missing?! I feel like an idiot right now. @Altius Premier Tutor
SOLUTION:
D is the correct answer. Counting the number of hydrogens, nitrogens, and oxygens in the molecule of uracil the molecular formula is C4H4N2O2. Since each of these numbers can be divided by two the empirical formula must be C2H2N2O2 making D the correct answer. Answer A is not correct as the empirical formula is incorrect. Answer B is showing both options as empirical formulas. Answer C gives both options as the molecular formula.
