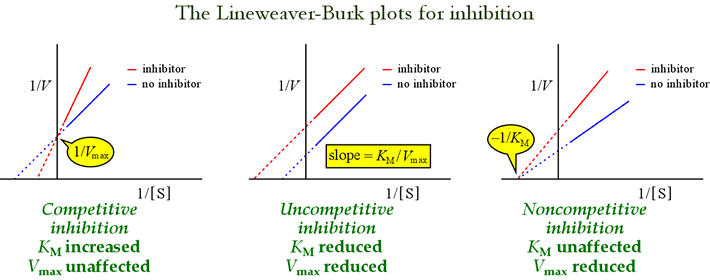
So, I have the basics memorized and understood:
a high KM = low affinity
a low KM = high affinity
Vmax is the max rate/velocity
KM is the concentration of substrate where it is 1/2 vmax
competitive inhibitor: KM increases, Vmax stays the same, slope increases. Vmax doesn't change because if the concentration of substrate is high enough, there is very little chance for the competitive inhibitor to bind to the active site. And Km increases because you need a higher substrate concentration.
noncompetitive inhibitor: KM stays the same, Vmax decreases, slope increases. Noncompetitive inhibitor binds an allosteric site, so no change in substrate concentration; however, Vmax decreases, because it binds so strongly that no amount of substrate can remove it. It is as if the enzyme bound to noncompetitive inhibitor is no longer there.
uncompetitive inhibitor: KM & Vmax both decrease, slope increases. Binds to the ES complex, decreasing both Vmax and KM.
--------------
Any suggestions or tweaks on understanding the above concepts any further?
Also, I'm having a hard time understanding the whole inverse y-axis and x-axis thing.
Why are the slopes increasing when an inhibitor is added?
a high KM = low affinity
a low KM = high affinity
Vmax is the max rate/velocity
KM is the concentration of substrate where it is 1/2 vmax
competitive inhibitor: KM increases, Vmax stays the same, slope increases. Vmax doesn't change because if the concentration of substrate is high enough, there is very little chance for the competitive inhibitor to bind to the active site. And Km increases because you need a higher substrate concentration.
noncompetitive inhibitor: KM stays the same, Vmax decreases, slope increases. Noncompetitive inhibitor binds an allosteric site, so no change in substrate concentration; however, Vmax decreases, because it binds so strongly that no amount of substrate can remove it. It is as if the enzyme bound to noncompetitive inhibitor is no longer there.
uncompetitive inhibitor: KM & Vmax both decrease, slope increases. Binds to the ES complex, decreasing both Vmax and KM.
--------------
Any suggestions or tweaks on understanding the above concepts any further?
Also, I'm having a hard time understanding the whole inverse y-axis and x-axis thing.
Why are the slopes increasing when an inhibitor is added?