- Joined
- Jan 10, 2017
- Messages
- 12
- Reaction score
- 3
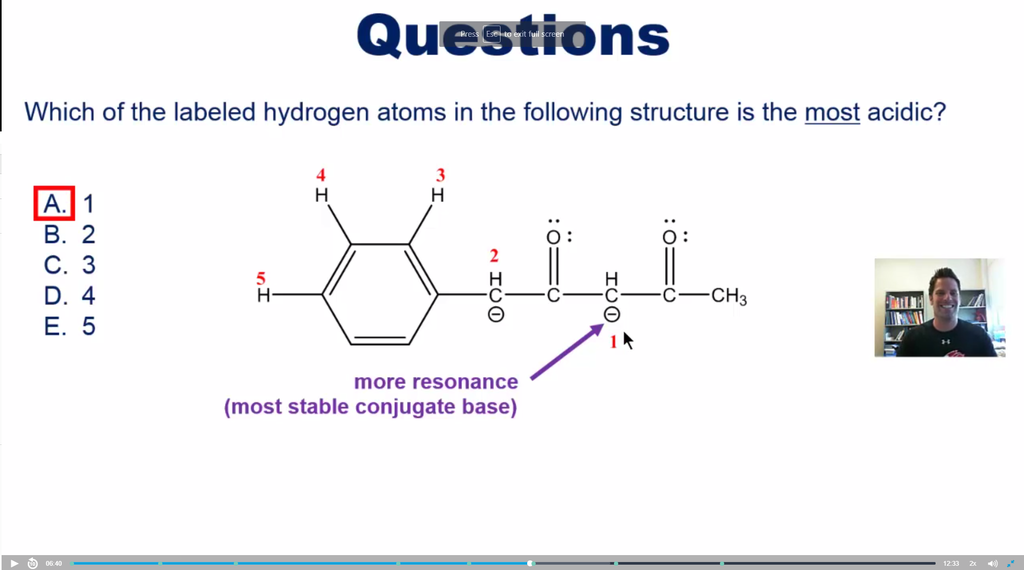
Hey guys, so Mike mentioned that Hydrogen #1 is the most acidic, however I thought that H #2 was more acidic because of benzene's resonance. Does a benzene + Oxygen beat out having two Oxygens when it comes to resonance stabilizing?