- Joined
- Dec 1, 2011
- Messages
- 18,579
- Reaction score
- 57
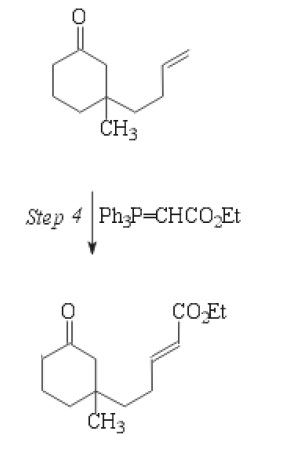
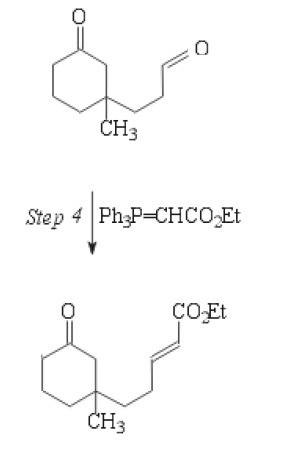
I thought the wittig reaction was dependent on a phosphonium ylide with a partial negative carbon attached to it. How does this ylide work for wittig?
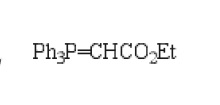
How does the carbonyl carbon end up with a partial negative if it has a partial positive due to the oxygens?
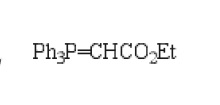
How does the carbonyl carbon end up with a partial negative if it has a partial positive due to the oxygens?