- Joined
- Apr 29, 2011
- Messages
- 2,171
- Reaction score
- 863
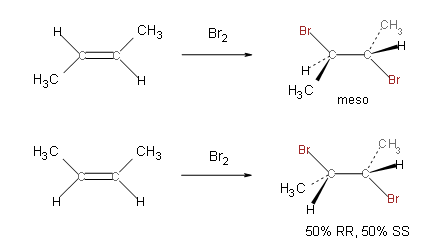
Let's say you have 1-butene. If you do FRH, why is the product (according to TBR*):
1-bromobutane
Why does the Br add to the alkene (as opposed to one of the normal sp3 carbons)?
Also, why does FRH in alkenes favor adding the halide to the the less substituted side?
*I can't seem to find the page at this moment but this is from what I remember
1-bromobutane
Why does the Br add to the alkene (as opposed to one of the normal sp3 carbons)?
Also, why does FRH in alkenes favor adding the halide to the the less substituted side?
*I can't seem to find the page at this moment but this is from what I remember