If a pure enantiomer undergoes free radical halogenation by abstraction of a hydrogen atom from its chiral carbon, racemization occurs, and the reaction produces a racemic mixture of the two possible enantiomers. What is (are) the product(s) when a pure enantiomer of 1-chloro-2-methylbutane undergoes such a reaction?
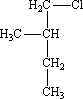
1-chloro-2-methylbutane
 A.
A.
I only

Correct Answer
 B.
B.
II only
 C.
C.
III only
 D.
D.
I and II only
Explanation:
A. The only products formed are the two enantiomers of 1,2-dichloro-2-methylbutane.
Can anyone explain why (±)-1,3-dichloro-2-methylbutane couldn't form? I understand it's C2 is tertiary and is favored, but why can't C3 form a secondary carbocation? The product would also be racemic.
edit: in fact, C3 should be more selective than C2. Since radical halogenation rate for Cl is 5:4:1 for tert, sec, and primary carbocation, and there are 2 H's on C3, and one 1 H on C2. So selectivity for C3:C2 should be 8:5 favoring C3. Is this just a really bad question, or am I missing something?

1-chloro-2-methylbutane
- (±)-1,2-dichloro-2-methylbutane
- (±)-1,3-dichloro-2-methylbutane
- (±)-1,4-dichloro-2-methylbutane

I only

Correct Answer

II only

III only

I and II only
Explanation:
A. The only products formed are the two enantiomers of 1,2-dichloro-2-methylbutane.
Can anyone explain why (±)-1,3-dichloro-2-methylbutane couldn't form? I understand it's C2 is tertiary and is favored, but why can't C3 form a secondary carbocation? The product would also be racemic.
edit: in fact, C3 should be more selective than C2. Since radical halogenation rate for Cl is 5:4:1 for tert, sec, and primary carbocation, and there are 2 H's on C3, and one 1 H on C2. So selectivity for C3:C2 should be 8:5 favoring C3. Is this just a really bad question, or am I missing something?
