Warning: huge wall of text. Most of it is nitpicky with regards to the scope of the MCAT. Suffice it to say that water diffuses from areas of low
solute concentration to areas of high
solute concentration; saying that water diffuses from areas of high
water concentration to areas of low
water concentration isn't entirely correct.
If the solutes decrease by 10 percent, the water will increase by 90% in a given volume. In that context, the inverse relationship seems valid.
This is not true. Let me try and clarify your current understanding: I imagine that you're picturing a blank sheet of paper as representing water. This blank sheet of paper represents pure water. Now, if we want to add solute, in your model, we would take a hole puncher and punch holes in that blank sheet of paper; each hole represents an amount of solute, so more holes means more solute. By this model, the more paper we have per area, the less holes we have per area, and the more holes we have per area, the less paper we have per area; thus, water and solute concentration would follow an inverse relationship by this model. Does this illustrate what you're thinking?
Aqueous solutions do not follow this hole-in-paper model. In fact, in some cases, adding solute to water can
increase the concentration of water. Why is this? First, we will have to talk about the two states a water molecule can occupy in solution:
1.
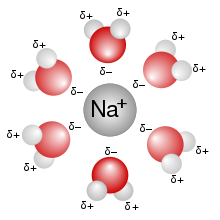
Bound: a water molecule can be a part of a hydration shell around an ion. A hydration shell is a shell of water molecules that surrounds an ion. So when we dissolve NaCl in water, NaCl will dissociate into Na+ and Cl-, and hydration shells will form around all the sodium ions and around all the chloride ions. The driving forces for the formation of these hydration shells are electrostatic interactions. In this picture,
you can see a hydration shell that has formed around a sodium ion; the negative poles of water (oxygen) coordinate with the positive sodium ion. A hydration shell around a chloride ion would have the positive poles of water (hydrogen) coordinate with the negative chloride ion. Because of these favorable electrostatic interactions, a water molecule arranged in a hydration shell is in a lower energy state than in the "free" water molecules not in a hydration shell:
2.
Free: a water molecule can be "free" in that it is not a part of a hydration shell. In this picture, the "free" water molecules are the grey ones labeled as "bulk water (random arrangement)."
(This picture also shows that a hydration shell can be several molecules thick. No need to worry about this.)
(Also note that free and bound water molecules are in a dynamic equilibrium with each other; they can swap places. No need to worry about this too much.)
Free water molecules, because they are not bound to solute molecules (as the ones in hydration shells are), are free to diffuse around. These are the
free water molecules I was talking about in my previous post.
Anyways, bound water molecules that are arranged in hydration shells take up less volume, because they are packed more efficiently. On the other hand, free water molecules--which, as you can see in the picture, are randomly arranged--are not as efficiently packed and so take up more volume. Thus, a solution in which little water is free (i.e., a lot of water is bound in hydration shells) may occupy a smaller volume than a solution in which a lot of water is free (i.e., not much water is bound in hydration shells). This is why the hole-in-paper model doesn't hold here.
(This is why adding NaCl to a cup of water won't change the solution's volume that much; while a volume of aqueous NaCl is added to the cup of water, the volume of the solvent is reduced, because bound water molecules in hydration shells are more tightly packed.)
Anyways, as we increase the solute concentration of a solution, the number of
free water molecules generally decreases, because it takes more water molecules to form hydration shells around an increased amount of solute. Thus, in areas of high solute concentration, we will have a low concentration of
free water, and in areas of low solute concentration, we will have a high concentration of
free water. Finally, because there is a higher concentration of free water where there is a lower solute concentration, free water will diffuse from areas of low solute concentration to areas of high solute concentration. Free water is free to diffuse, whereas bound water is not, so it's important to differentiate between the
concentration of free water and the
concentration of water.
The
concentration of water is more nuanced and less helpful in predicting osmotic equilibria. For example, take the bucket example. We are going to assume that each bucket has the same number of moles of water and the same number of moles of solutes, though different solutes.
Tell me where/if I'm confused here, but I'm going to expand on the bucket example. If the two buckets were separated by a semipermeable membrane, water would be free to move. Even though each side of the bucket could initially have slightly different volumes and different molarities, I think the water would move from the side with greater percentage of water molecules per volume to the side with less water molecules per volume. Am I wrong?
You are correct that the solutions in each bucket will have slightly different volumes and thus different molarities. The bucket with a smaller volume of solution will have a higher concentration of water and a higher concentration of solute; call this bucket A. The bucket with a larger volume of solution will have a lower concentration of water and a lower concentration of solute; call this bucket B. By your reasoning, water diffuses from bucket A to B, but that would not be the case. Water diffuses from areas of low solute concentration to areas of high solute concentration, so water diffuses from bucket B to A. This is why it's important to differentiate between the
concentration of free water and the
concentration of water. Bucket B has a higher concentration of
free water than bucket A, but bucket A has a higher concentration of water than bucket B; claiming that water diffuses from areas of high water concentration to areas of low water concentration would be wrong, because that would incorrectly predict that water diffuses from bucket A to B.
In reality, the difference in volumes between equimolar solutions might be negligible, so you wouldn't have to worry about this, especially on the MCAT,
unless the MCAT asked you a question that required knowing the difference between molarity and molality.
You are correct that the driving energy for diffusion is the kinetic energy inherent in those free water molecules. However, it's much easier to predict osmotic equilibria based on solute concentrations.