- Joined
- Mar 13, 2003
- Messages
- 2,187
- Reaction score
- 1,224
RV is of paramount importance. If you can't get blood to the LV (via RV ejection), then the LVAD will not work. When you start adding 1-2 l/m to the systemic circulation, that is 1-2 l/m extra flow that the RV suddenly is confronted with. It doesn't have a lot of time to accommodate for such a change in hemodynamics. Therefore, inhaled vasodilators and RV augmentation with volume and inotropes may be necessary. If these maneuvers don't work this may cause the RV to go into failure. One thing to keep in mind is that the RCA (supplies the RV) is anterior. Air tends to go down the RCA when coming off bypass. Furthermore, CPB doesn't protect the RV nearly as well as the LV. This only adds insult to injury when dealing with LVAD placement. Many of the the RVADS that are placed in practice are actually due to an LVAD that overwhelmed the RV leading to RV failure.
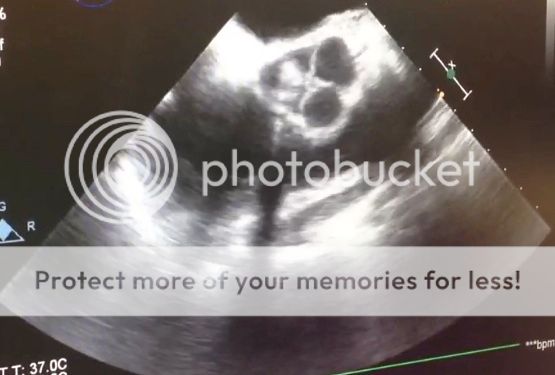
The "suckdown" phenomena is usually due to an underfiled LV causing the interventricular septum to get pulled towards the LV free wall. Doing this alters the geometry of the RV. Augmenting the geometry of the RV may equate to poor systolic performance AND tricuspid annulus dilatation leading to acute tricuspid regurgitation.
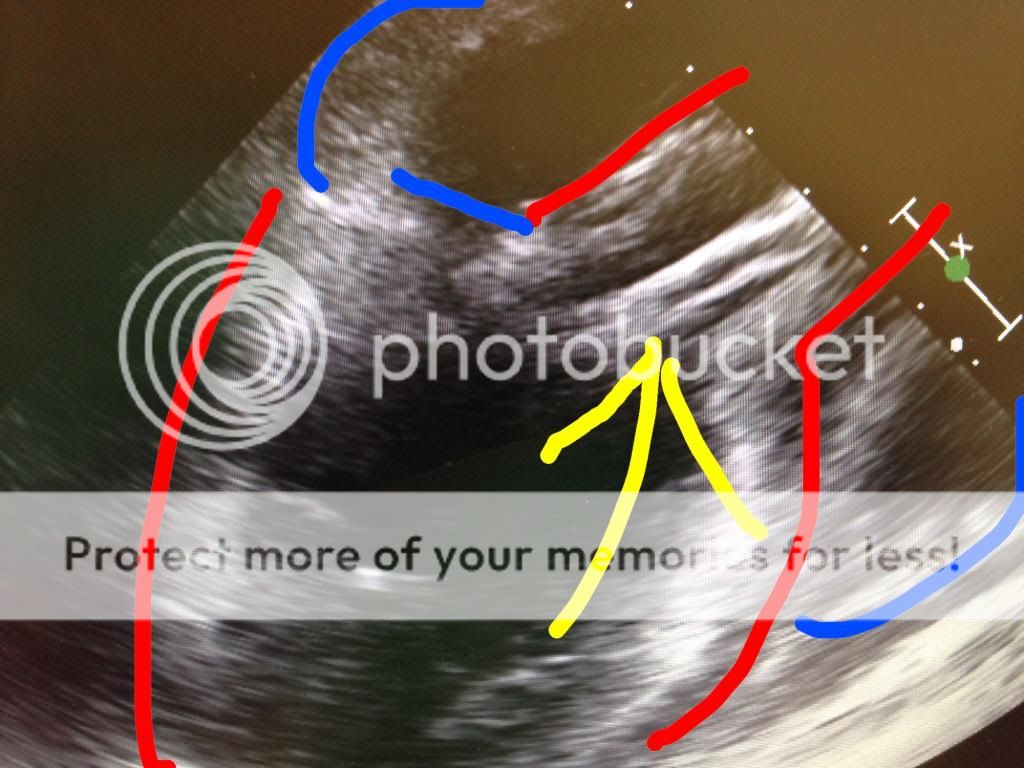
Here is a modified bicaval view showing this:
If you see this coming off bypass you need to think: oh... oh... what can we do to make this better.
The answer...? As weird as it sounds, reduce your flow rates on your LVAD and augment volume status. These maneuvers will return the RV to it's regular shape and hopefully increase RV unloading to the LV.
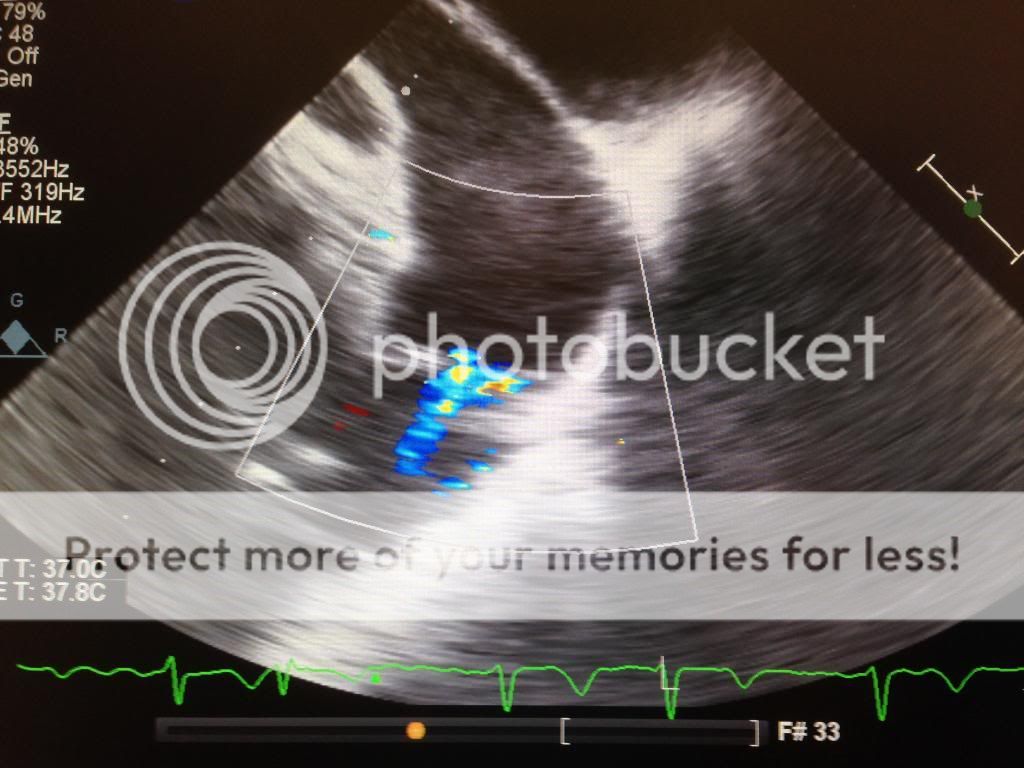
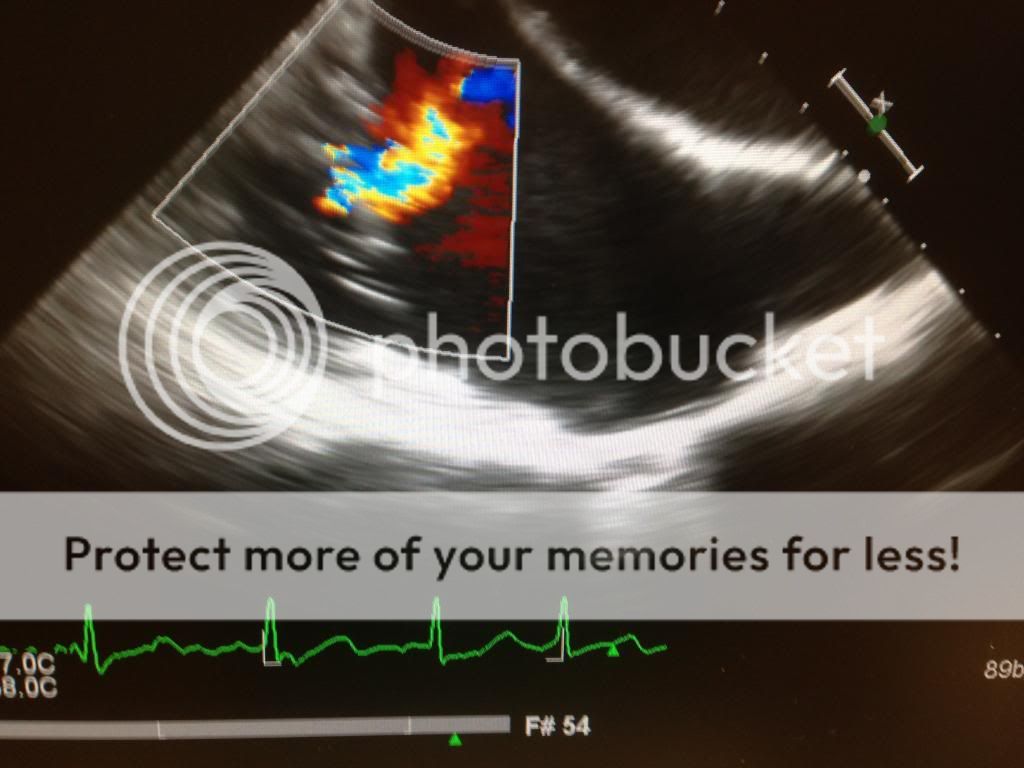
In addition to causing the suckdown phenomena, the inflow canulas can become obstructed. Check for this by using color Doppler and seeing laminar flow. You can also use CWD. Flows should be less than 2.5 m/s. Similarly, the outflow cannula (ascending aorta) should show slightly lower flows. 1-2 m/s.
AI is bad... LVAD worsens AI and therefore you may not achieve increased organ perfusion. You can create a partial closed loop from the ascending aorta back into the LV causing a volume overload problem. You need a competent valve. What are the choices?
1) Repair the valve.
2) Replace the valve and deal with higher morbidity/mortality. Never use mechanical valves. Always bioprosthetic ones.
3) Sounds crazy... but if it's destination therapy or bridge to transplant, you can surgically sew the aortic valve shut so that it doesn't open at all!
Good stuff.
Love LVAD physiology. I would add one more thing to the management of a suction event- vasopressin. Selectively increasing SVR without increasing PVR will help your RV, and along with decreasing the VAD flow, will help restore septal geometry.