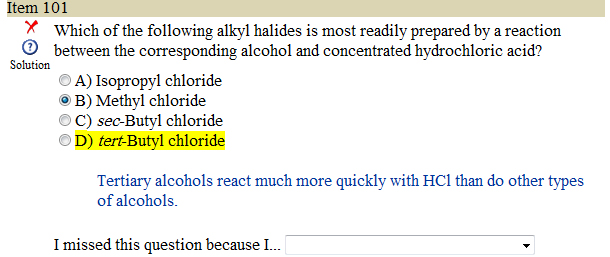sorry if this question has been answered already but i didn't see it so..
PS 27) Though 395-nm light is in the visible region of the electromagnetic spectrum, it is very near:
A. the radio wave region
B. the microwave region
C. the infrared region
D. the ultraviolet region <- Correct
Now if I had memorized the wavelengths of visible light these should have been super easy points. However, I didn't - so I went with info presented in the passage:
"Bromine is red-brown liquid that absorbs light very strongly at a wavelength of 395nm"
Using this info, I deduced that red light is at 395nm (incorrect), and therefore it is near the infrared region. I understand that this is a simple matter of memorization, but what is wrong with my reasoning as per info in the passage? Should I disregard passage info next time in favor of textbook info I know is correct?
Thanks.
Well, I don't think you should discard passage information. From what I can tell, things are in the passage for a reason, and if the passage gives numbers and equations, get ready to use them.
In your case, I think you might have read the information incorrectly. It was stated that Bromine absorbs light very strongly at 395nm, so it seems to me that it would show up with a strong, red color. It is red because the light you see is reflected.
Reflected color: Reflected color is color you can't see in the dark without another source of light (for example, a red apple). So, if a substance absorbs near 400, then it's color is the opposite (near 700 nm, or simply red). Do a search on google for the color wheel, and you might get an understanding of what I mean. So if something absorbs around the 700nm range, then it will probably appear green/blue (near the 400 nm range).
Emitted Color: Emitted color is color you can see in the dark, because it is emitting color and light. An example is a red firecracker. It appears red because it emitts red light (700nm) and this light that hits your eyes is not reflecting of anything. It is emitted at 700 nm and you see it as 700 nm
It's best to memorize the range
UV...400nm (blue)---------700nm (red)....IR
And know that the 400 nm is lower in wavelength, but higher in frequency and that the 700 nm is higher in wavelength, but lower in frequency. So the higher the frequency, the more damaging the light source (that's why too much UV radiation is not good for you).
Hope this helps pal!!