- Joined
- Jul 28, 2014
- Messages
- 1,982
- Reaction score
- 5,648
The question asks you to find how many stereoisomers of this compound exist. The formula is 2^n where n is number of stereocenters. The answer they want is 2^5 = 32.
I don't understand why the atoms marked in red are not stereocenters? A nitrogen bound to three different groups and a lone pair should have tetrahedral shape and be chiral, no?
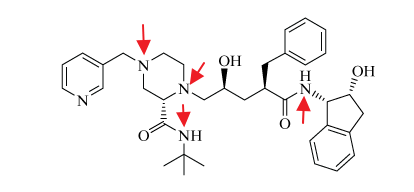
I don't understand why the atoms marked in red are not stereocenters? A nitrogen bound to three different groups and a lone pair should have tetrahedral shape and be chiral, no?
