- Joined
- Feb 23, 2015
- Messages
- 400
- Reaction score
- 373
The following picture shows the confusion I have about the following alkyne hydration reactions:
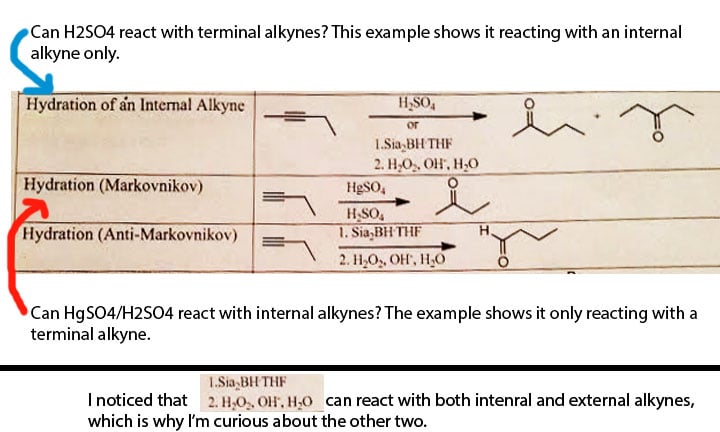
These reactions are from Chad's reaction list and I tried to check the road maps in the Ochem Destroyer but couldn't find these.
Can anyone verify if alkyne location is critical to their success?
These reactions are from Chad's reaction list and I tried to check the road maps in the Ochem Destroyer but couldn't find these.
Can anyone verify if alkyne location is critical to their success?
