- Joined
- Jun 15, 2013
- Messages
- 87
- Reaction score
- 7
When questions refer to hydrogen bonding, what do they mean exactly? I mean, what consists of "H bonding"? I get confused because technically esters can accept H bonds because they have a lone pair, but they are aprotic and are unable to donate H bonds. So overall, can we say that esters CAN participate in hydrogen bonding? When questions refer to H bonding do they mean that the compound must be BOTH H bond donor AND acceptor to be considered to be able to hydrogen bond?
I've come across this ambiguity in a couple of questions from different resources and each consider H bonding differently. i.e one case considers a lone pair as being able to H bond and the other resource doesn't refer to this.
An example.... (SPOILER ALERT: QUESTION FROM KAPLAN FL 3R!!)

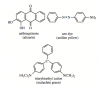
Initially I picked C (the correct answer) but then thought about this ambiguity and picked D because I thought that the tertiary amine has a lone pair and *technically* can participate in H-bonding.. so it should have at least some adherence... I also didn't thing steric hinderance would be in issue
I've come across this ambiguity in a couple of questions from different resources and each consider H bonding differently. i.e one case considers a lone pair as being able to H bond and the other resource doesn't refer to this.
An example.... (SPOILER ALERT: QUESTION FROM KAPLAN FL 3R!!)
Initially I picked C (the correct answer) but then thought about this ambiguity and picked D because I thought that the tertiary amine has a lone pair and *technically* can participate in H-bonding.. so it should have at least some adherence... I also didn't thing steric hinderance would be in issue