I'm confused about two statements made in the Kaplan books:
"Anions always move towards the anode,
and cations always move toward the cathode."
"Electrons always flow through the wire from the anode to the cathode
and current (protons) flows from cathode to anode."
But isn't an electron an anion?
And isn't a proton a cation? :/
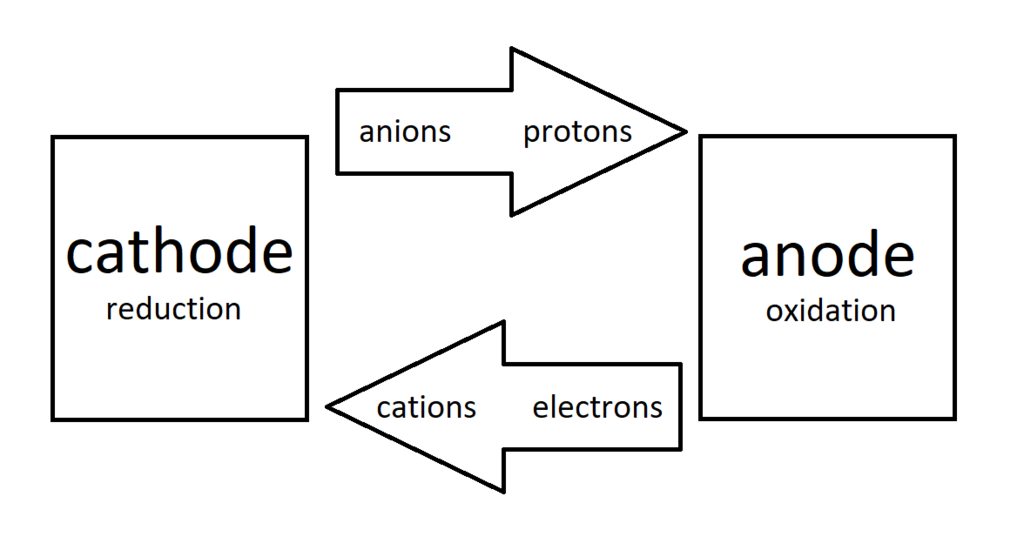
"Anions always move towards the anode,
and cations always move toward the cathode."
"Electrons always flow through the wire from the anode to the cathode
and current (protons) flows from cathode to anode."
But isn't an electron an anion?
And isn't a proton a cation? :/
