- Joined
- Sep 2, 2014
- Messages
- 858
- Reaction score
- 548
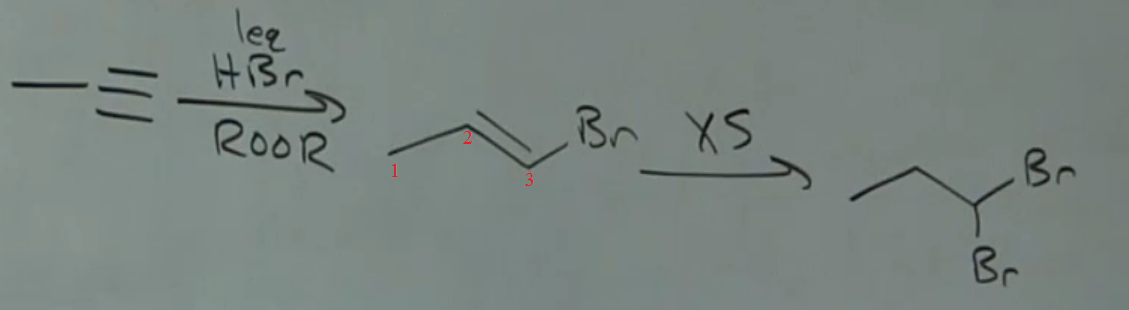
Why did second anti-Mark rxn add H to #2 when both 1&2 equally substituted?
Last edited:


Why did H get added to C2 instead of C3? Help!
This is poorly written.....confusing indeed. Assuming the author of the problem meant excess HBr/ ROOR.....it was simply 2 consecutive anti-Markovnikov additions. Anti-Markovnikov addition only works when HBr/ROOR is used. This reaction is very very important to know, and is used in organic synthetic procedures on a wide scale.
Hope this helps.
Dr. Romano
The radical does not for on the carbon with the bromine. It's more stable there because it is on the secondary carbon, not the primary carbon.
Take a look at the mechanism. Hope it helps.
View attachment 194712
