- Joined
- Jun 30, 2014
- Messages
- 59
- Reaction score
- 30
I recently took a practice test from the Princeton Review and one a passage based question asked,
"Which of the following elements is a result of the decay of the 90Sr released by atmospheric nuclear weapons testing?"
In the passage it stated that Sr decayed by beta decay. I didn't know whether to choose that it decayed by beta plus decay or beta minus decay. Both were answer choices. The solution afterwards says,
" It is safe to conclude that this unqualified beta emission is in fact β–emission. The nuclear decay reaction is therefore
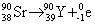 ."
."
Do any of you have an idea as to why it can be assumed that unspecified beta decay is indeed beta minus decay as apposed to beta plus decay? One of the answer choices was 90Rb, so I wasn't sure which to choose and their explination didn't tell me why they assumed beta minus decay.
I appreciate the help!
"Which of the following elements is a result of the decay of the 90Sr released by atmospheric nuclear weapons testing?"
In the passage it stated that Sr decayed by beta decay. I didn't know whether to choose that it decayed by beta plus decay or beta minus decay. Both were answer choices. The solution afterwards says,
" It is safe to conclude that this unqualified beta emission is in fact β–emission. The nuclear decay reaction is therefore
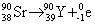
Do any of you have an idea as to why it can be assumed that unspecified beta decay is indeed beta minus decay as apposed to beta plus decay? One of the answer choices was 90Rb, so I wasn't sure which to choose and their explination didn't tell me why they assumed beta minus decay.
I appreciate the help!
