- Joined
- Jul 7, 2013
- Messages
- 617
- Reaction score
- 162
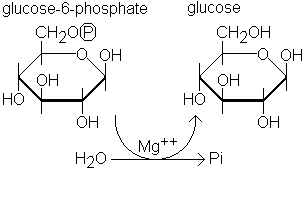
Not a fan of Lehninger. The diagrams are convoluted. I don't like looking at Haworth projections and Fischer projections. Why can't there be more chairs and line-angle diagrams like in any organic chemistry text? Sure, I could convert the Haworths and Fischers to line-angle forms as I'm used to after taking two semesters of organic chemistry ... but I'd rather not do this. Even the professor draws chairs and line-angle diagrams in class, so having to go through the book and seeing something that looks totally different is jarring.
So, is there any biochem text written with line-angle diagrams and chairs?
So, is there any biochem text written with line-angle diagrams and chairs?