cT3 is a factor that pops in the majority of all guidelines.
A recurrence in a reconstructed breast results in the worst cometic outcome of all. How big is the risk of recurrence (locally) without RT? What do you think?
I'd say 10%, perhaps even up to 15%.
Pneumonitis with RNI in MA20 was 1,2%.
But hey, who is talking about ENI? Have we agreed to give ENI?
What is worse?
A
potentially deadly chest wall recurrence or a necrosis in a reconstructed breast or a grade III pneumonitis? I have made up my mind. You can go ahead and ask the patient.
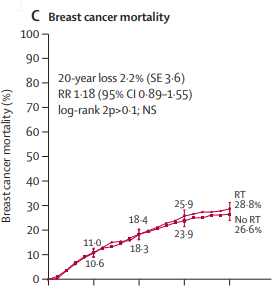
Who says the risk of recurrence is 3-5% in the chest wall 1% in the axilla in "thousands of patients".
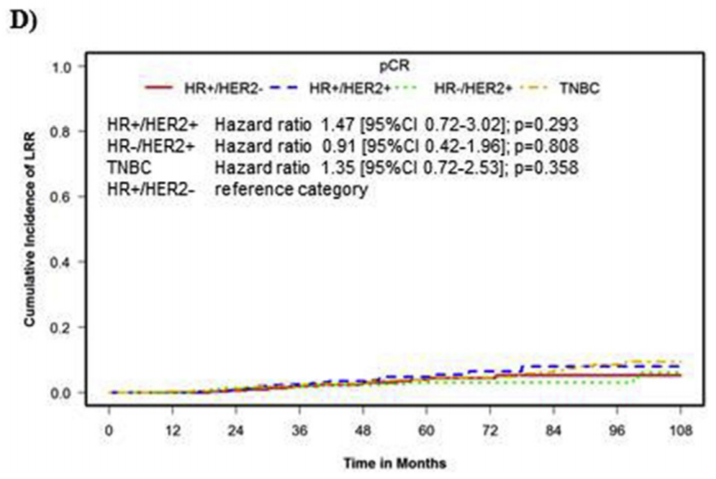
The Mamounas paper from 2012:
View attachment 308188
16 patients with cT1-3 cN0 tumors and pCR after neoadjuvant chemotherapy. That's the data you have...
And they had 6.2% recurrences without RT (which is basically 1 patient out of 16!).
Please bear in mind that both NSABP-18 & -27 included full axillary dissection for all patients, not SLNB.
I leave it up for discussion whether or not SLNB after NAC in a 6cm (practically) tripple negative tumor is the "right" way to go to assess the axilla (if you are not planning to irradiate)...