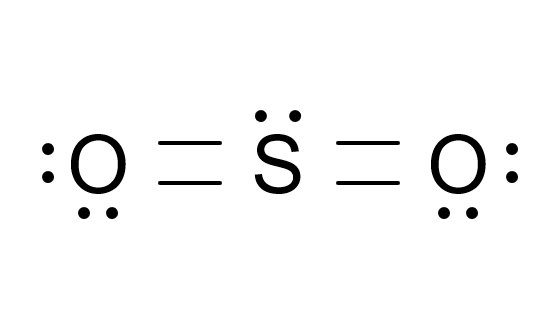D
dentalstudent2021
https://qph.is.quoracdn.net/main-qimg-fa998b5f9a9fb166b810c3d2f1388666?convert_to_webp=true
confused as to why this is often not shown as the structure for SO2 ?
Thanks.
confused as to why this is often not shown as the structure for SO2 ?
Thanks.