TBR General Chemistry Section 9 Kinetics, Passage VII, #46.
There appears to be conflict between what the passage says and the answer key says.
46. Addition of water to the reaction would have what effect?
A. It would increase the reaction rate.
B. It would have no effect on the reaction rate.
C. It would decrease the reaction rate.
D. It would make the reaction third-order.
Answer: C
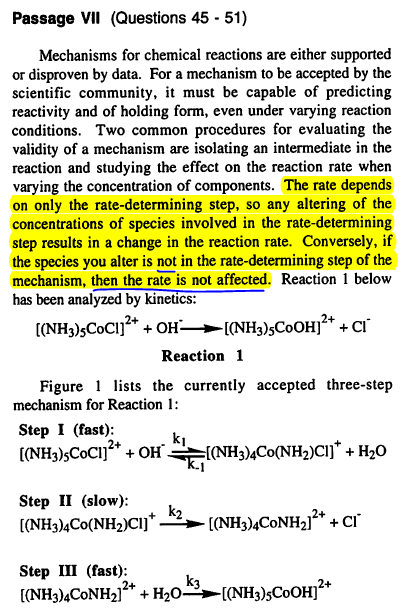
What I highlighted in the passage says that if the species is not in the rate determining step, altering its concentration shouldn't affect the reaction rate. The answer explanation says that adding water DOES affect reaction rate, even though water isn't in the rate determining step. Anyone want to clarify why adding water affects the rate? I'm not totally understanding their reasoning. #49 is a similar case, adding OH- (increasing pH) somehow makes reaction rate go up, so it's not just this problem that is weirding me out.
#49 is a similar case, adding OH- (increasing pH) somehow makes reaction rate go up, so it's not just this problem that is weirding me out.
EDIT: May have reasoning as to why the bolded stuff is true at the bottom of the thread.
There appears to be conflict between what the passage says and the answer key says.

46. Addition of water to the reaction would have what effect?
A. It would increase the reaction rate.
B. It would have no effect on the reaction rate.
C. It would decrease the reaction rate.
D. It would make the reaction third-order.
Answer: C
What I highlighted in the passage says that if the species is not in the rate determining step, altering its concentration shouldn't affect the reaction rate. The answer explanation says that adding water DOES affect reaction rate, even though water isn't in the rate determining step. Anyone want to clarify why adding water affects the rate? I'm not totally understanding their reasoning.
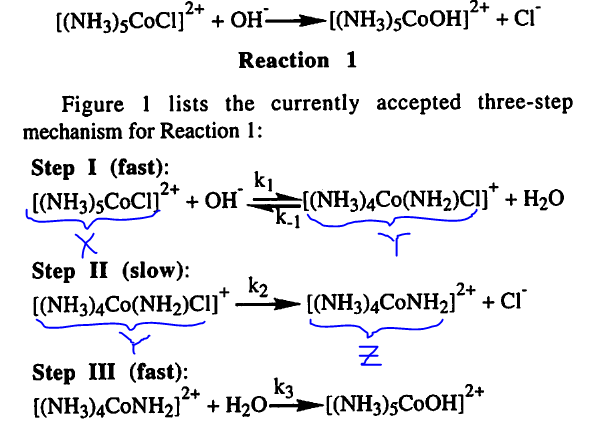
Choice C is correct. Adding water to the reaction mixture would push the Step I reaction backwards, generating more reactants ([(NH3)5CoCl]2+ and OH") and consuming the intermediate product ([(NH3)4Co(NH2)Cl]+). The rate-determining step, Step II, depends on the concentration of the product from Step I [(NH3)4Co(NH2)Cl]+. Thus, a decrease in products from Step I slows down the rate-determining step, thereby decreasing the overall reaction rate. The best answer is choice C. Choose C for sensations of correctness and satisfaction.
EDIT: May have reasoning as to why the bolded stuff is true at the bottom of the thread.
Last edited: