- Joined
- Jun 3, 2014
- Messages
- 109
- Reaction score
- 18
- Points
- 4,631
- Pre-Medical
Hi, I was wondering if anyone could help explain this problem to me? I found Kaplan's explanation confusing and lacking. I am utterly lost on this one.
Here is the necessary information from Kaplan Kinetics Test 1 to answer #16 and #17
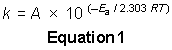
Here are the questions, answers, and Kaplan's explanation
#16
What is the activation energy for Reaction 1?
A - 9.71 kJ/mole
B - 22.4 kJ/mole
C - 80.7 kJ/mole
D - 186 kJ/mole (CORRECT)
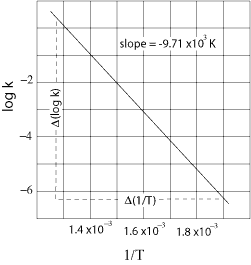
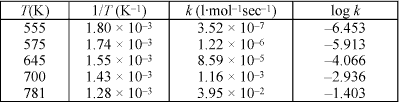
If you didn't know how to read Figure 1, this question would be rather difficult to answer. Keep in mind that the MCAT incorporates graphs and data tables into their passages, and you can count on questions like this one. Anyway, Figure 1 is a plot of the log of the rate constant versus the inverse of the temperature. You are also given the slope of the line. Where do you see a relationship between the rate constant and the temperature? You see it in Equation 1, the Arrhenius equation. What you need to realize is that Figure 1 is a plot of Equation 1. If you take the log of both sides of Equation 1, you get that the log of k is equal to the log of A - Ea divided by the product of 2.303, the ideal gas constant, and the temperature. (As you can see, you must know how to manipulate logarithms, too.) This is in the form of the general equation for a straight line: y is equal to mx + b, where b is the y intercept and m is the slope. Because x = 1/T, the slope of this line is equal to -Ea divided by the product of 2.303 and the ideal gas constant, and you are given the numerical value of the slope at the top of Figure 1. So, all you need to do is solve for the activation energy, Ea, and approximate the answer, since the choices are pretty spread out.
m = -Ea/2.303R
-9.71 x 103 K= -Ea/2.303 x 8.314 J mol-1K-1
-10 x 103 (20 J mol-1) = -Ea
200 x 103 J mol-1 = 200 kJ mol-1 = Ea
Choice D is the closest and is, therefore, the correct choice.
(A) Miscalculation. This answer choice is the result of misinterpreting the relationship between Equation 1 and Figure 1. This answer choice suggests that the slope is equal to -Ea, when in fact the slope is really equal to -Ea/2.303R.
(B) Miscalculation. This answer choice is the result of guessing and/or random errors in calculation. If you guessed this answer choice, subconsciously you may have been remembering that 22.4 is an important number in gases, as it's the volume of one mol of gas at STP.
(C) Miscalculation. This answer choice is the result of random errors in calculation.
#17
In Figure 1, what does the intercept with the y-axis represent?
A - log A (CORRECT)
B - log K
C - Ea
D - something i can't remember
The logarithmic form of the Arrhenius equation, log k = log A - Ea/2.303RT is in the form of the general equation of the straight line, y = mx + b. y is equal to the log of k, m (as we determined in the last question) is equal to -Ea/2.303R, and x is equal to 1/T. That leaves b as equal to the log of A; thus, Choice A is the correct answer.
(B) Distortion. The log of k is equal to y, not the y intercept.
(C) Distortion. -Ea is merely part of the slope.
(D) Distortion. This expression really does not correspond to anything.
Thanks!!!
Here is the necessary information from Kaplan Kinetics Test 1 to answer #16 and #17

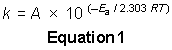

Here are the questions, answers, and Kaplan's explanation
#16
What is the activation energy for Reaction 1?
A - 9.71 kJ/mole
B - 22.4 kJ/mole
C - 80.7 kJ/mole
D - 186 kJ/mole (CORRECT)
If you didn't know how to read Figure 1, this question would be rather difficult to answer. Keep in mind that the MCAT incorporates graphs and data tables into their passages, and you can count on questions like this one. Anyway, Figure 1 is a plot of the log of the rate constant versus the inverse of the temperature. You are also given the slope of the line. Where do you see a relationship between the rate constant and the temperature? You see it in Equation 1, the Arrhenius equation. What you need to realize is that Figure 1 is a plot of Equation 1. If you take the log of both sides of Equation 1, you get that the log of k is equal to the log of A - Ea divided by the product of 2.303, the ideal gas constant, and the temperature. (As you can see, you must know how to manipulate logarithms, too.) This is in the form of the general equation for a straight line: y is equal to mx + b, where b is the y intercept and m is the slope. Because x = 1/T, the slope of this line is equal to -Ea divided by the product of 2.303 and the ideal gas constant, and you are given the numerical value of the slope at the top of Figure 1. So, all you need to do is solve for the activation energy, Ea, and approximate the answer, since the choices are pretty spread out.
m = -Ea/2.303R
-9.71 x 103 K= -Ea/2.303 x 8.314 J mol-1K-1
-10 x 103 (20 J mol-1) = -Ea
200 x 103 J mol-1 = 200 kJ mol-1 = Ea
Choice D is the closest and is, therefore, the correct choice.
(A) Miscalculation. This answer choice is the result of misinterpreting the relationship between Equation 1 and Figure 1. This answer choice suggests that the slope is equal to -Ea, when in fact the slope is really equal to -Ea/2.303R.
(B) Miscalculation. This answer choice is the result of guessing and/or random errors in calculation. If you guessed this answer choice, subconsciously you may have been remembering that 22.4 is an important number in gases, as it's the volume of one mol of gas at STP.
(C) Miscalculation. This answer choice is the result of random errors in calculation.
#17
In Figure 1, what does the intercept with the y-axis represent?
A - log A (CORRECT)
B - log K
C - Ea
D - something i can't remember
The logarithmic form of the Arrhenius equation, log k = log A - Ea/2.303RT is in the form of the general equation of the straight line, y = mx + b. y is equal to the log of k, m (as we determined in the last question) is equal to -Ea/2.303R, and x is equal to 1/T. That leaves b as equal to the log of A; thus, Choice A is the correct answer.
(B) Distortion. The log of k is equal to y, not the y intercept.
(C) Distortion. -Ea is merely part of the slope.
(D) Distortion. This expression really does not correspond to anything.
Thanks!!!
Last edited:
