- Joined
- Jan 29, 2008
- Messages
- 935
- Reaction score
- 3
From Chad's Video's:
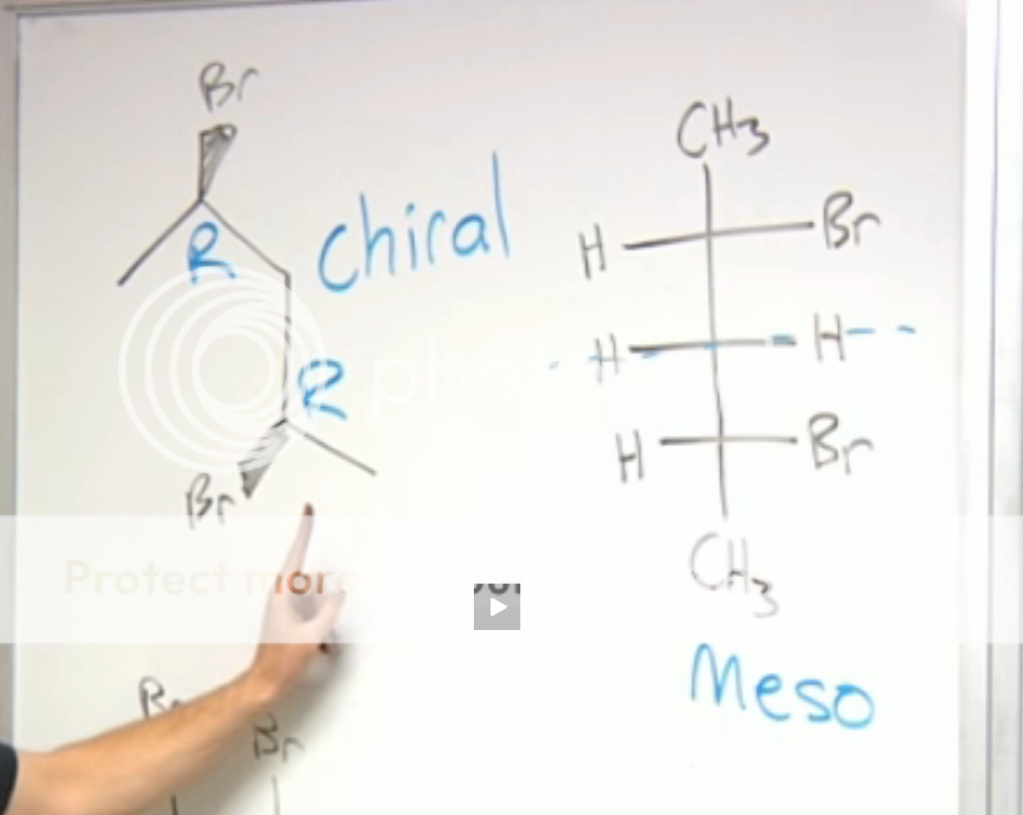
The molecule on the right, as pictured, is R,R; however, if you rotate C3, wouldn't that flip the Br behind the plane and make it R,S?

The molecule on the right, as pictured, is R,R; however, if you rotate C3, wouldn't that flip the Br behind the plane and make it R,S?

 to both of us.
to both of us.