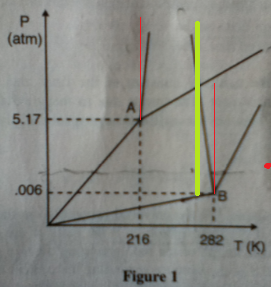
The following is a combined phase diagram diagram for materials A and B.
1.) Which of the following would have its volume reduced if the pressure is increased?
A. Material A at its triple point
B. Material B at its triple point
C. Material A at 1000 K and 1 atm
D. Material B at 273 K and 0.006 atm
Answer: C Riiiiiiiight.

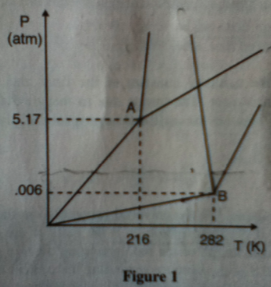
1.) Which of the following would have its volume reduced if the pressure is increased?
A. Material A at its triple point
B. Material B at its triple point
C. Material A at 1000 K and 1 atm
D. Material B at 273 K and 0.006 atm
Answer: C Riiiiiiiight.