Here's my take on this...
The authors state:
"A radiotherapy decision attestation sheet was completed before randomisation documenting provider intent as to whether radiotherapy would be offered or not, the general treatment fields (whole pelvis
vs prostate bed alone), intent to provide androgen deprivation, and duration of such therapy, so that decisions before versus after PET could be compared systematically."
Now, the authors avoided putting all these treatment decisions in a graph or text and really show how all these decisions were implemented in both groups.
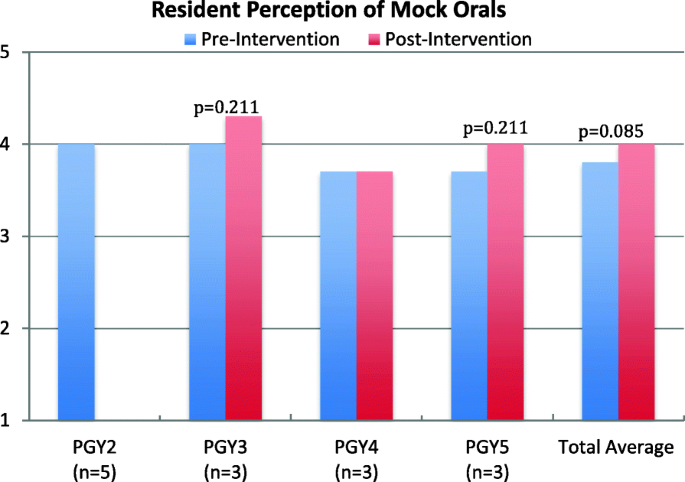
A chart in the Appendix shows some decision changes in the PET-group,
Initially it seems that 45 patients were scheduled to undergo prostate bed RT and 34 patients pelvic RT.
Then, post-PET 31/45 got prostate bed RT and 14/45 got pelvic RT. So, the PET lead to a treatment intensification for about roughly a third of the patients scheduled to undergo prostate bed RT (they got the pelvis on-top, because they were cN1 on PET). At the same time, 10/34 patients scheduled to undergo pelvic RT got prostate bed RT (because the PET came back cN0) and 4/34 got nothing, since they were metastatic.
In the non-PET group 56 patients received a prostate bed RT and 25 received pelvic RT.
Unfortunately, apart from the general statement of how many patients received ADT and for how long, no further information was provided on whether or not changes occured compared to baseline. I presume, that a physician would be more willing to give ADT if he saw disease in the pelvis in the PET scan or he may ommit it if he saw nothing. Did ADT prescription intent change after the PET? We do not know.
It's striking to see the imbalance among the groups.
Already from the start, it seems that the non-PET group was scheduled to undergo considerably less treatment than the PET group.
In the non-PET group: 70% received prostate-bed only RT "at physicians choice".
In the PET-group 56% were scheduled to undergo pelvic RT from the start and then that number grew to 69% post-PET.
It is important to state that 17-19% of all patients in both groups had pN1 disease and received pelvic RT irrespective of PET or physicians choice.
So there is an unclear imbalance from the start and I do not see why the investigators did not stratify for that!
To explain the difference in event free survival between the groups the authors suggest that more precise staging with PET helped to increase treatment volumes and treat patient with pelvic disease that would otherwise be left untreated. True, 14 more patients received pelvic RT in that group than initially scheduled. At the same time however 14 patients also experienced deescalation of their treatment based on PET.
Can we really tell if omitting pelvic RT in PET-negative patients is a good idea? Can PET accurately rule out nodal disease? Do not forget the recently published
paper on PET-cN0 high-risk patients from India who benefitted from pelvic RT...
This trial is being "sold" as a trial of PET, but actually it's certainly also a trial of "pelvic RT vs. prostate bed RT" in two very unbalanced groups in my humble view...