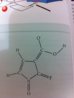
What would be formal charge on the double bonded Fluorine and the single bonded Oxygen? Thanks!

You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Formal charge help needed!
- Thread starter sdp07
- Start date
- Joined
- Jul 7, 2013
- Messages
- 617
- Reaction score
- 162
Depends on what else is attached to both atoms. Your picture is broken.
- Joined
- Feb 18, 2014
- Messages
- 220
- Reaction score
- 50
Fluorine makes one bond, oxygen makes two bonds, confused what u talking aboutWhat would be formal charge on the double bonded Fluorine and the single bonded Oxygen? Thanks!

- Joined
- Jul 7, 2013
- Messages
- 617
- Reaction score
- 162
I can't see your picture... but unless double bonded F is usu. +1 and single bonded O is –1
If single bonded, F is always 0. Not sure where you pulled +1 from.
- Joined
- Nov 27, 2013
- Messages
- 89
- Reaction score
- 61
? I was taught that F is always -1 when dealing with oxidation numbers. Unless you're talking about F2?If single bonded, F is always 0. Not sure where you pulled +1 from.
- Joined
- Jul 7, 2013
- Messages
- 617
- Reaction score
- 162
This thread is about formal charge.
Also in diatomic fluorine the oxidation state of fluorine is 0. Not +1.
Also in diatomic fluorine the oxidation state of fluorine is 0. Not +1.
- Joined
- Feb 18, 2014
- Messages
- 220
- Reaction score
- 50
I guess the he is saying once the fluorine make two bonds with the other atoms, then there is a plus charge on the fluorine, but this is highly unlikely. Fluorine is highly electronegative by itself, a positive charge on fluorine is highly unstableIf single bonded, F is always 0. Not sure where you pulled +1 from.
- Joined
- Jul 7, 2013
- Messages
- 617
- Reaction score
- 162
There's a positive formal charge yes. Let's be specific about charge. There probably isn't going to be say a positive partial charge too.
- Joined
- Feb 18, 2014
- Messages
- 220
- Reaction score
- 50
for this molecule, O=-1 and f=+1, N=+1, the overall charge of the molecule is +1, the charge separation in this case is stabilized by resonance, or conjugation (some people would like to use this term to describe the effect more precisely)
- Joined
- Jul 7, 2013
- Messages
- 617
- Reaction score
- 162
for this molecule, O=-1 and f=+1, N=+1, the overall charge of the molecule is +1
I hope you mean the single-bonded O.
- Joined
- Feb 18, 2014
- Messages
- 220
- Reaction score
- 50
Yup, the only oxygen that makes one bondI hope you mean the single-bonded O.
Similar threads
- Replies
- 3
- Views
- 9K