Just do a quick check with the answer.
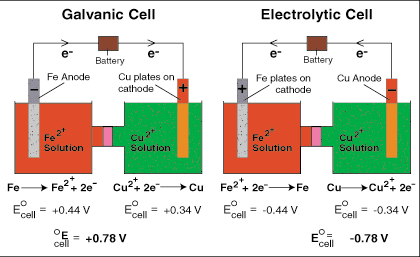
If they provide reduction potentials, you should know that the more negative a reduction potential is, the more likely it is going to be oxidized, while the more positive a reduction potential is, the more likely it is going to be reduced. Now, if they give you any reaction and you figure out which species is being oxidized and which one is being reduced, ask yourself, with the table they provided, does molecule X usually become oxidized or reduced according to their reduction potentials? If the species is becoming reduced and you know that it usually will be oxidized, you know that the E standard of cell is going to be (-) and the reaction is going to be nonspontaneous, standard G = (+)
Example
Zn+2(aq) + 2e--->Zn(s) E standard= (-0.76)
Cu+2(aq) + 2e---->Cu(s) E standard = (+0.34)
The reduction potential of Zn 2+ is -0.76 and that of Cu+2 is 0.34. You know that Zn likes to be oxidized in this case and Cu likes to be reduced. If however, the reaction you are given, provided in the question stem, Zn is reduced and Cu is oxidized, this is a nonspontaneous reaction and powered by an outside source - i.e. battery, so that the reaction may occur, meaning E standard of cell should come out (-)
Remember, E standard of cell is always opposite of standard gibbs free energy G.