- Joined
- Aug 6, 2007
- Messages
- 553
- Reaction score
- 5
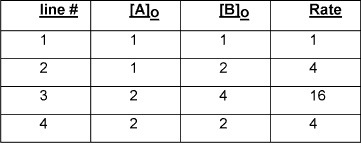
How did they add an extra row? I don't understand the explanation.
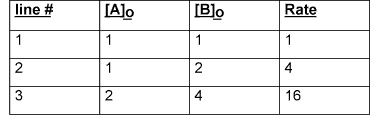
Looking at lines 1 and 2, where the concentration of reactant A is held constant, we see that a doubling in the concentration of B results in a quadrupling of the rate, therefore the reaction is second order with respect to B. We can now look at lines 2 and 3 (or 1 and 3) to determine the required reaction order. From line 2 to line 3, the concentration of B is doubled; the reaction rate should thus quadruple due to the influence of the concentration increase of second order reactant B. We can divide out this quadrupling effect and create a hypothetical line 4: