- Joined
- Dec 18, 2015
- Messages
- 3,216
- Reaction score
- 4,930
ASTRO 2018, ~500-person trial (w/ Namenda), "practice changing," cognitive failure reduced by ~8% at 6 mos (~68%->60%), p=0.03
ESTRO 2019, ~160-person trial (? Namenda), at 4 months no difference (p=1.0) and at 8 months ~8% difference (p=0.46)
(Trials w/ p-values in 0.02-0.05 range have about a 25-50% replication failure rate.)
Perhaps p<0.005 should be the new standard for "practice-changing" new standards of care...
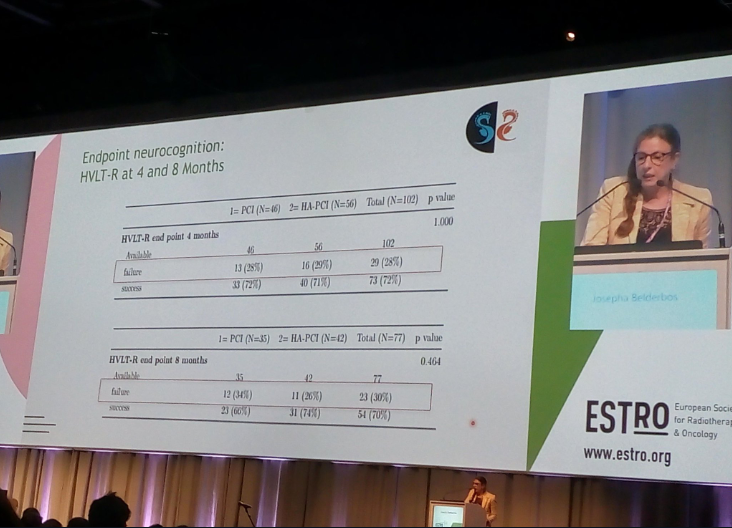
ESTRO 2019, ~160-person trial (? Namenda), at 4 months no difference (p=1.0) and at 8 months ~8% difference (p=0.46)
(Trials w/ p-values in 0.02-0.05 range have about a 25-50% replication failure rate.)
Perhaps p<0.005 should be the new standard for "practice-changing" new standards of care...

