6
663697
This is Q41 from TPR test 3.
They say that p-TSA is an acid and don't mention the presence of a base. When I wrote out the mechanism I got an imine, so am I supposed to assume that water will act as a base to abstract a proton to produce the C=C bond?
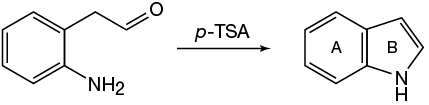
They say that p-TSA is an acid and don't mention the presence of a base. When I wrote out the mechanism I got an imine, so am I supposed to assume that water will act as a base to abstract a proton to produce the C=C bond?

Last edited by a moderator:
