- Joined
- Jan 5, 2015
- Messages
- 146
- Reaction score
- 38
Can someone please tell me why this reaction is an exergonic reaction...
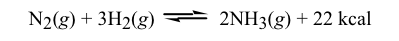
Can someone please tell me why this reaction is an exergonic reaction...View attachment 192142
Glad we could help!Ya it's the Haber process.
Thanks guys, appreciate the help!
In an exergonic process, we see an exothermic reaction......we clearly have one here......since heat is given off. An exergonic reaction also has a NEGATIVE Gibbs Free energy. Here, we must be careful,,,,,,the entropy is decreasing.......since less gas molecules are being made. Thus.....the entropy and enthalpy terms are both NEGATIVE. This means it will be spontaneous at LOW temperature.......Assuming you are at 25 degrees Celsius .....the reaction would be termed EXERGONIC. At higher temperatures, this would not be true, since the reaction would be favored in the reverse direction.
Hope this helps.
Dr. Jim Romano
Hi Dr. Romano,
I wanted to clarify and make sure I really understood everything...
Where I got confused is that I didn't realize +22kcal was written IN the reaction as a product, which means it's an exothermic reaction. For this reason, I should assume that the reaction is taking place at 25 degrees Celsius because an exothermic reaction with a negative deltaS requires the reaction to take place at low temperatures.
But if it was written as deltaH = +22kcal, heat would be considered as a reactant which means the reaction is endothermic, also leading me to believe the reaction is taking place at high temperatures because deltaS is still negative.
Finally, as you stated, all of this means that an exothermic reaction is always an exergonic process and vice-versa (same with endothermic rxn = endergonic process).
An exothermic reaction does NOT guarantee that a reaction is exergonic. An exergonic reaction means a reaction is exothermic and spontaneous. There are some reactions that are exothermic but not spontaneous, so you have to be careful here. If heat is written on the left side of the equation it means the reaction is endothermic. If a reaction is endothermic and has a positive entropy it could be spontaneous if at high temperatures.
Hope this helps
Dr. Romano
