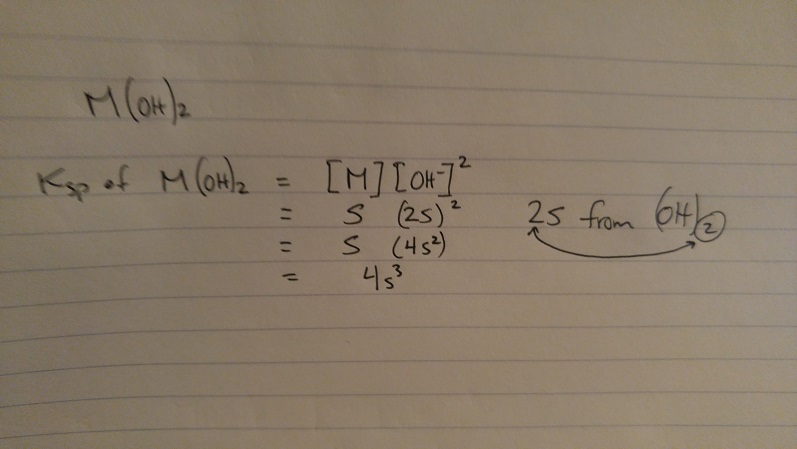7
752779
Hi Guys, I'm confused how they solve for Ksp valvue
Ksp = S(2s)^2
= S(4s^2)
Ksp = 4S^3
I don't get the second step how did they end up with S(4S^2)? and what happen to [M]?
Please help
Thanks


Ksp = S(2s)^2
= S(4s^2)
Ksp = 4S^3
I don't get the second step how did they end up with S(4S^2)? and what happen to [M]?
Please help
Thanks