- Joined
- Oct 22, 2015
- Messages
- 780
- Reaction score
- 1,325
- Points
- 5,796
Advertisement - Members don't see this ad
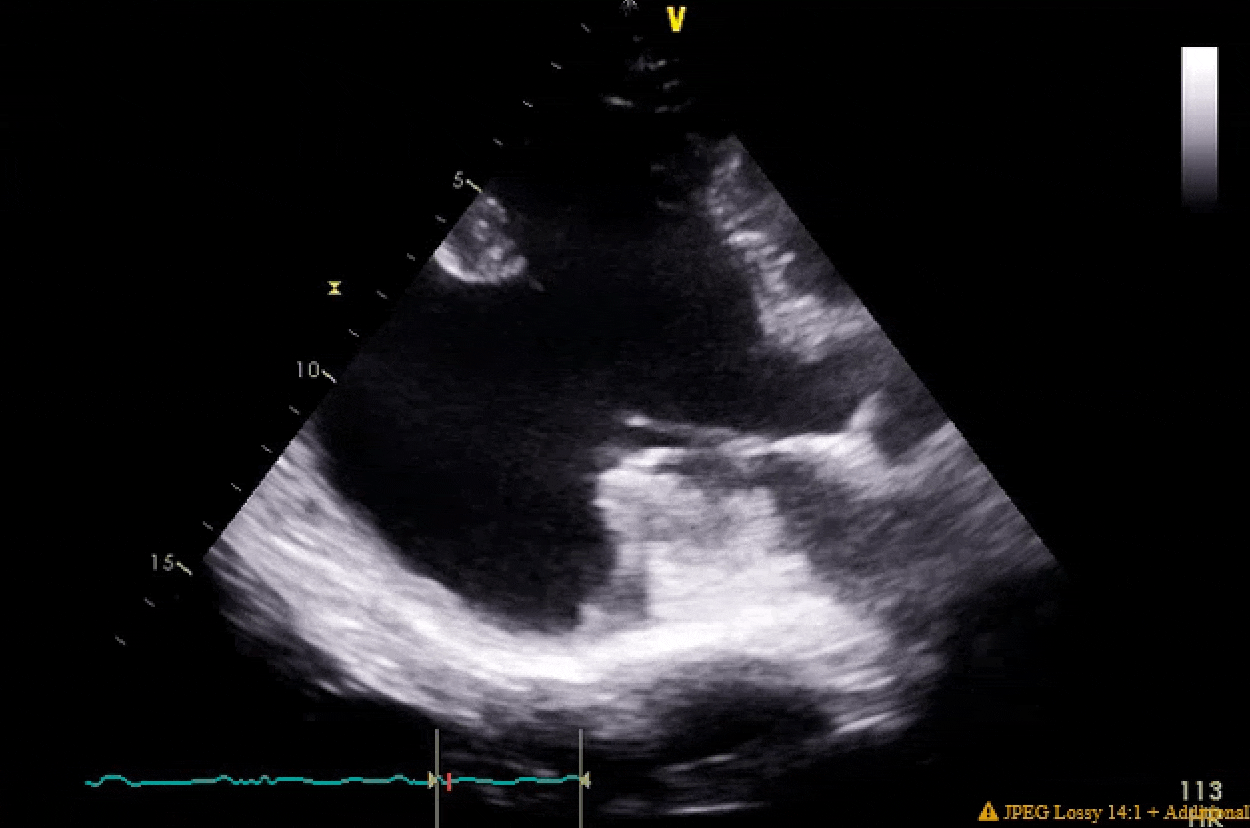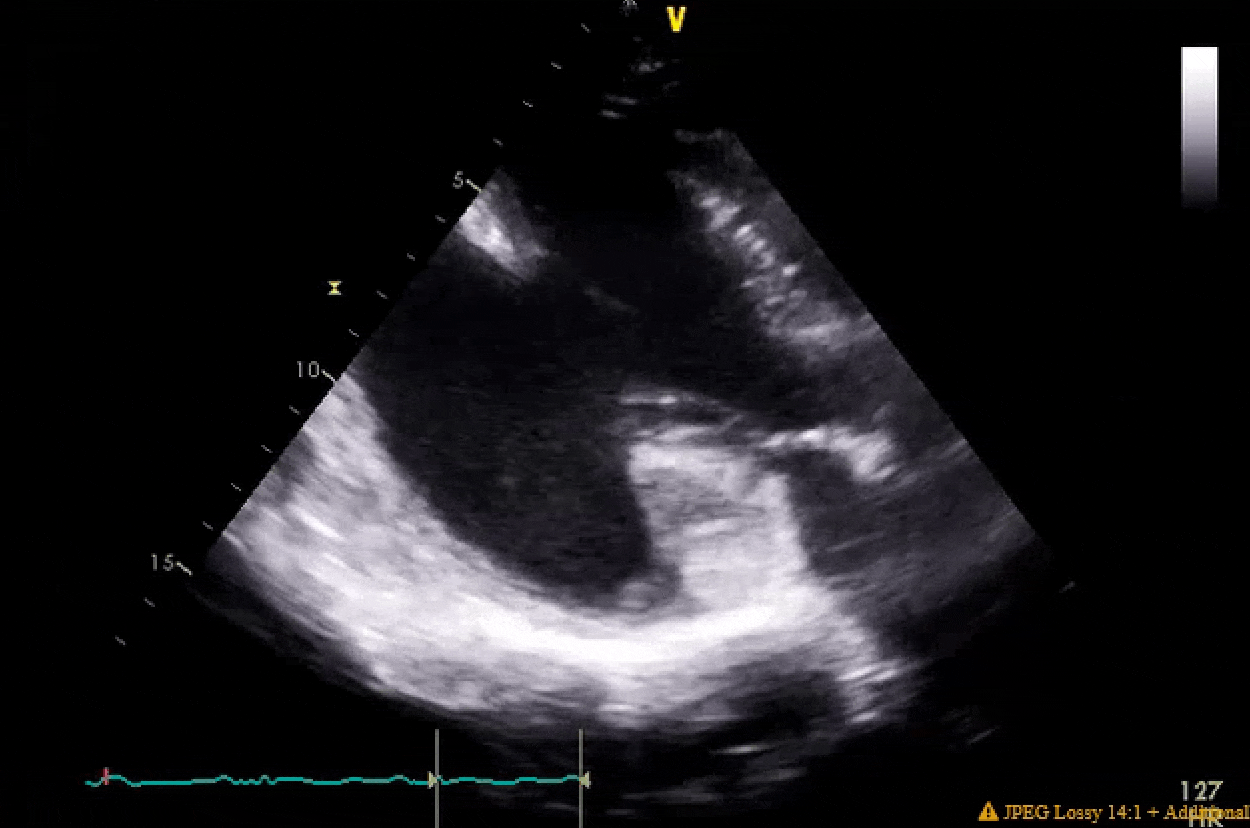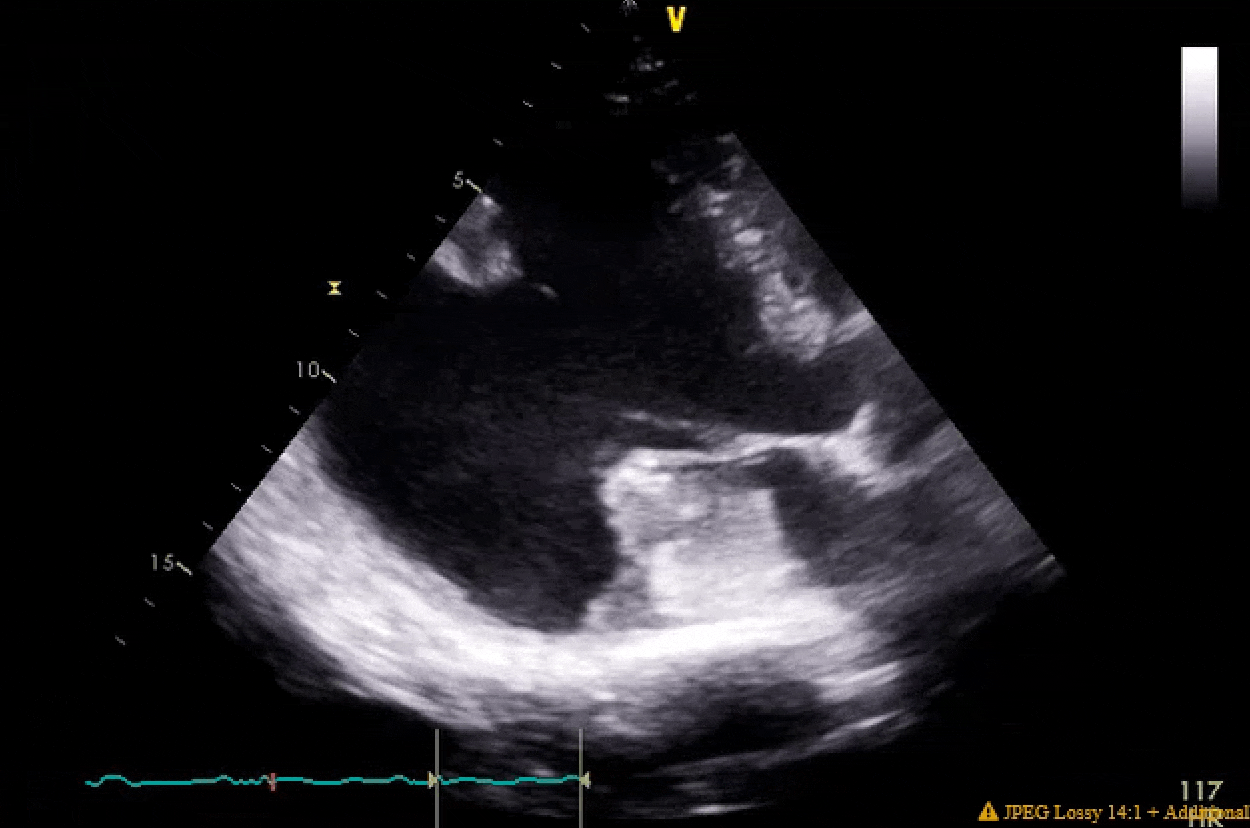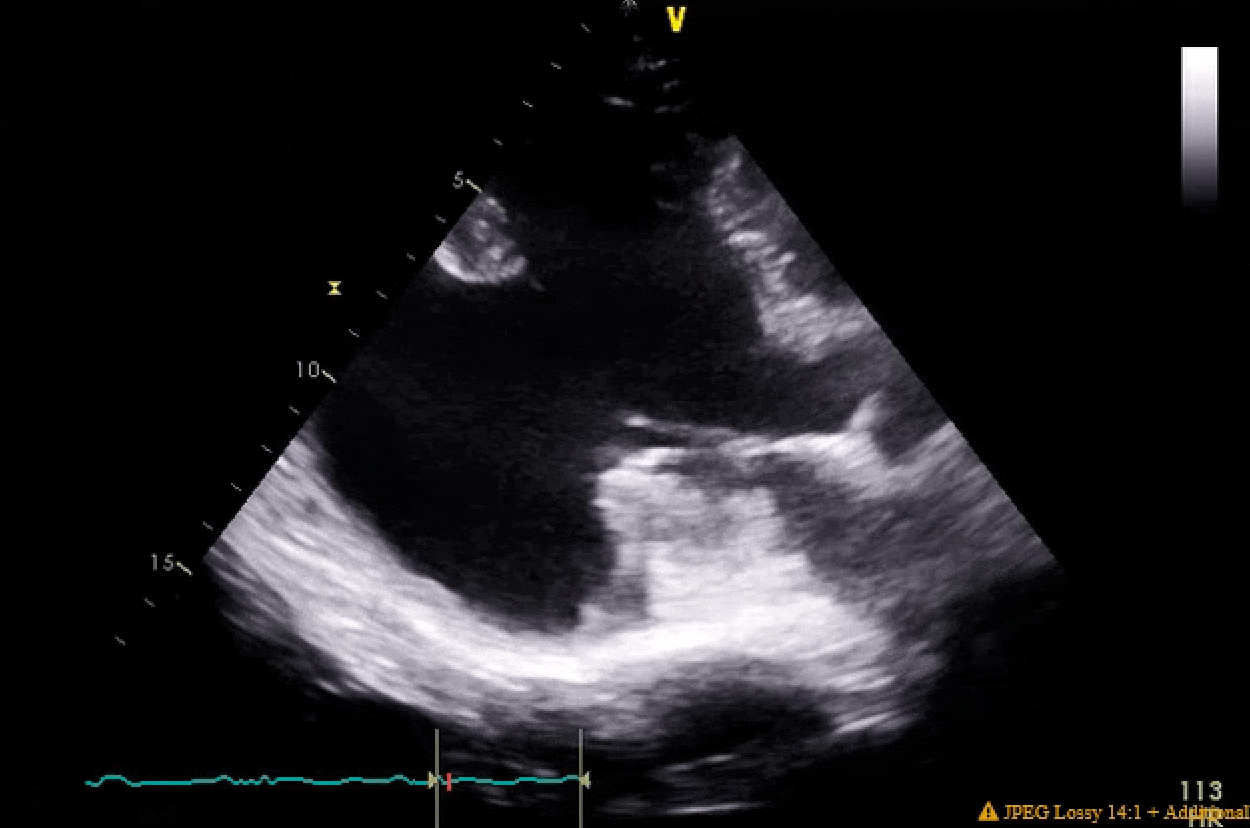
Pretty much spot on. The gifs didn't capture the way I would've liked (they're kinda choppy), but if you squint real hard you'll notice in the 4ch color shot that there is a central jet and a highly, eccentric anteriorly directed jet. There ended up being a cleft plus myxomatous degeneration leading to both Carpentier type I and II mechanisms.
You'll also notice the LV function is pretty bad pre-pump, and absolutely atrocious post-pump. The RV function is also moderately to severely depressed post-pump. Both the clamp time and pump run were very short, MVR went without a hitch. I expressed to my resident that this case highlighted the difference between doing pump cases for revascularization in ischemic cardiomyopathy with viable myocardium vs doing valvular surgery for people with non-ischemic cardiomyopathy. In the literature, AMI cardiogenic shock does better than decompensated NICM cardiogenic shock, and I find that to be generally (and anecdotally) true in the OR as well when one has post-cardiotomy shock.
The post-pump LV clip with an EF of ~15% is with the pt on high dose epi, dobutamine, milrinone, iNO, levo, vaso and IABP with a barely acceptable MAP and CI. He went on to require a takeback to the cathlab a few hours after he arrived in the ICU for impella placement due to worsening shock, and that highlighted some of the system issues we face with having surgeons who do not routinely put in their own impellas, VA-ECMO, LV vents, etc. They tried to punt on this case (either for transfer or possible clip), but pressure from cards was heavy given this was a young man (about age 50) with good functional status.
Finally, these clips highlight that in some patients you are just not going to be able to get a classic valentine heart 4 chamber view. I tried every combination of omniplane and flexion but his cardiomegaly and rotation made it impossible get a clip where I wasn't severely obliquely cutting through the LA. People btch a lot about the quality of clips in the aPTE, but difficult views are not terribly infrequent IRL.
Patient needed a VAD not a mitral intervention. Big hearts with **** function getting on pumps to fix a mitral lesion is a good case of attempted murder. The fact that he was 50 is even more criminal.