- Joined
- Jun 9, 2014
- Messages
- 1,443
- Reaction score
- 771
Hi everyone!
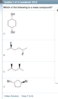
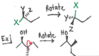
I'm confused on why this answer is a meso compound. The requirements to be meso are having a chiral center, and a plane of symmetry, but with a 6- carbon chain, how is that possible?
Thanks as always!
I'm confused on why this answer is a meso compound. The requirements to be meso are having a chiral center, and a plane of symmetry, but with a 6- carbon chain, how is that possible?
Thanks as always!