- Joined
- Dec 2, 2015
- Messages
- 438
- Reaction score
- 23
Why isn't this molecule a meso compound? Can't you draw a plane of symmetry from SH to oh?
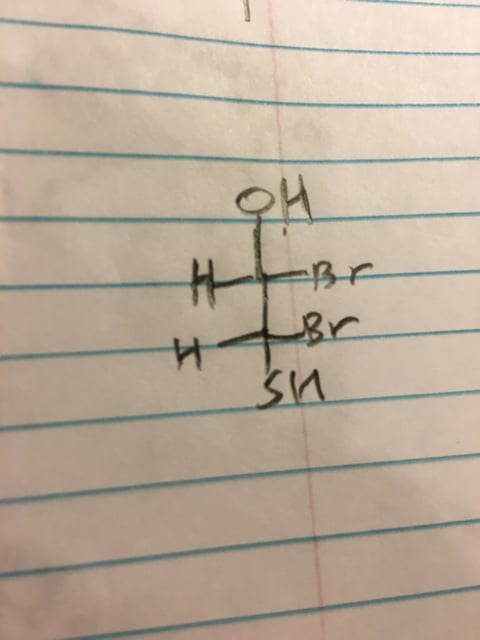
Sent from my iPhone using SDN mobile
Sent from my iPhone using SDN mobile
