- Joined
- Feb 16, 2012
- Messages
- 34
- Reaction score
- 1
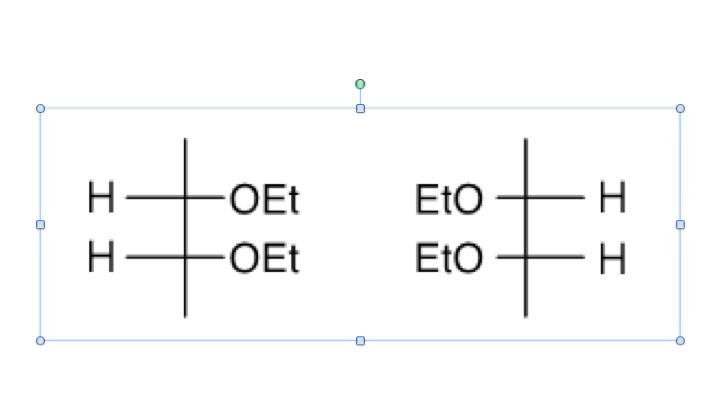
Hi all, I just wanted to ask if somebody can help me clarify meso compounds vs identical compounds. This is one problem from bootcamp: I understand that it is identical since it has mirror-image and mirror-plane of symmetry and it has enantiomers, if assigned R and S. Left compound would have (top-bottom) S,R and right compound would have (top-bottom) R,S.
So it says that one way to identify identical compounds from meso-compounds is identical have enantiomers and meso-compounds has no enantiomers. Therefore if just looking at one compound lets say the left compound and ignoring the right compound, this compound will be meso-compound with mirror-plane of symmetry correct?
Next, if I flipped the right compound, with EtO pointing at the right and comparing this to the original left compound with the OEt pointing at the right, the compounds will be meso-compound, because when I assign R and S (top-bottom), it will be: S,R and S,R. It will have no enantiomers and have mirror of symmetry and superimposable. I am I correct? I am really trying to determine meso from identical compounds. Thanks.

So it says that one way to identify identical compounds from meso-compounds is identical have enantiomers and meso-compounds has no enantiomers. Therefore if just looking at one compound lets say the left compound and ignoring the right compound, this compound will be meso-compound with mirror-plane of symmetry correct?
Next, if I flipped the right compound, with EtO pointing at the right and comparing this to the original left compound with the OEt pointing at the right, the compounds will be meso-compound, because when I assign R and S (top-bottom), it will be: S,R and S,R. It will have no enantiomers and have mirror of symmetry and superimposable. I am I correct? I am really trying to determine meso from identical compounds. Thanks.

